In this essay we will discuss about:- 1. Essay on Air Pollution 2. Essay on the Principal Types of Air Pollutants 3. Essay on the Factors Affecting Air Pollution 4. Essay on the Causes of Air Pollution 5. Essay on the Effects of Air Pollution 6. Essay on the Dangers of Air Pollution 7. Essay on the Control Measures to Check Air Pollution 8. Essay on Prevention and Control. Read this essay to learn about: – Essay on air pollution 350 words, Air pollution essay introduction, Causes of air pollution essay, Air pollution paragraph essay, Essay about air pollution cause and effect, Paragraph on air pollution in English, Air pollution essay conclusion!
Essay Contents:
- Essay on Air Pollution
- Essay on the Principal Types of Air Pollutants
- Essay on the Factors Affecting Air Pollution
- Essay on the Causes of Air Pollution
- Essay on the Effects of Air Pollution
- Essay on the Dangers of Air Pollution
- Essay on the Control Measures to Check Air Pollution
- Essay on the Prevention and Control of Air Pollution
1. Essay on Air Pollution:
An air pollutant is known as a substance in the air that can cause harm to humans and the environment. Pollutants can be in the form of solid particles, liquid droplets, or gases. In addition, they may be natural or man-made.
Air pollution is a phenomenon by which particles (solid or liquid) and gases contaminate the environment. Such contamination can result in health effects on the population, which might be either chronic (arising from long-term exposure), or acute (due to accidents).
Other effects of pollution include damage to materials (e.g., the marble statues on the Parthenon are corroded as a result of air pollution in the city of Athens), agricultural damage (such as reduced crop yields and tree growth), impairment of visibility (tiny particles scatter light very efficiently), and even climate change (certain gases absorb energy emitted by the earth, leading to global warming).
Air pollution is certainly not a new phenomenon. Early references to it date back to the Middle Ages, when smoke from burning coal was already such a serious problem that in 1307 King Edward I banned its use in lime kilns in London. More recently, there have been major episodes of air pollution, such as the 1930 catastrophe in the Meuse Valley, Belgium, where SO2 and particulate matter, combined with a high relative humidity, caused sixty-three excess deaths in five days. In 1948 similar conditions in Donora, Pennsylvania, a small industrial city, caused twenty excess deaths in five days, and in the early 1950s in London, England, two episodes of “killer fogs” claimed the lives of more than 6,000 people.
Essay # 2. Principal Types of Air Pollutants:
The principal types of air pollutants are:
1. Gases:
The important gaseous pollutants in the air from different sources are CO2, CO, SO2, NOx, H2S, Cl2, Fluorine etc.
2. Chemicals:
Important chemicals which pollute the air are — Arsenic (As) from coal, oil furnaces, glass manufacturing and fertilizer plants (from HPC tower); benzene (C6H6) from refineries and motor vehicles; Cadmium (Cd) from smelters, burning waste, coal and oil furnaces; formaldehyde (HCHO) from motor vehicles and chemical plants; HCl from incinerators; HF from fertilizer plants and smelters; Hg from coal and oil furnaces and smelters; Manganese (Mn) from steel and power plants; Nickel (Ni) from smelters, coal and oil furnaces; Lead (Pb) from automobiles, smelters; SiF4 from chemical plants.
3. Secondary Air Pollutants:
Air pollutants directly released from any source into the environment are called primary air pollutants. For example — Soot released from unburned fuel, benzopyrene (hydrocarbon) released from cigarette smoke, CO, lead.
Secondary air pollutants are formed from primary pollutants under specific conditions.
Thorndike (1976) has given following examples of secondary pollutants:

4. Particulate Matters:
Particulate matters or particulates are fine solids or liquid droplets (suspended in air) with a diameter ranging between 0.02 – 1.00 µm. From health viewpoint, particles in the range of 0.02 to 10 µm are of significance. The large sized particulates ate grit— having a diameter over 500/µm.
Particulates in the air may be viable or nonviable. The viable particulates are the minute living organisms that are dispersed in the atmosphere viz., microbes like bacteria, fungi, moulds, algae etc. Among these, viable algae are most commonly found in the atmosphere. Fungi in air would be allergic to humans.
Non-viable particulates of importance are formed either by the breakdown of larger materials or by the condensation of minute particles and droplets.
Non-viable particulates in the atmosphere are of four principal types:
1. Mists
2. Smoke
3. Fumes, and
4. Dusts.
Mists and fumes are the liquid particulates whereas dust and smoke are solid particulates.
1. Mists:
These are liquid droplets suspended in air with a diameter <10 µm. Example of mist may be the portion of insecticides and herbicides that actually miss their targets and travel through the air. Mists are formed by the condensation of vapour. For example-
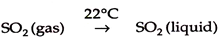
2. Smoke:
Smoke denotes very small soot particles produced by burning and combustion of organic matter. In other words, small sized particulates are smoke (gas-born solids) with particles usually less than 2.0 µm in diameter. Oil smoke, tobacco smoke and carbon smoke are typical examples of this type of particulate emission.
These are suspended solids in air less than 1.0 µm in diameter. The condensed vapours — fumes of metals — are the well-known particulates of this type. Examples of this category also include metallurgical fumes and alkali fumes.
These are generally solid, rarely liquid, particles suspended in air or some other gas with a diameter of 1.0 – 100 µm. Non-viable dust particulates in the atmosphere consist of ground limestone, sand tailings from floatation, pulverized coal, cement fly ash and silica dust.
Anthropogenic (man-made) dusts in the general environment originate chiefly from transportation, industrial and agricultural activities. Moreover, workers employed in specific occupation are exposed to high amounts of dusty particles.
Further particulates may be inorganic or organic type, called inorganic and organic particulate matter (OPM). OPM originates mainly from combustion of fuels, automobiles and vegetation. PAH (polycyclic aromatic hydrocarbons) like chrysene, benzo fluoroethane, benzo (a)-pyrene, benzidine etc. are some organic particulates of carcinogenic nature.
Gamma particles and radioactive nuclides like C-14, strontium 90, caesium-137 etc.
Transport through evaporation and exhaust emission of unburnt gasoline accounts for the major part of hydrocarbon pollutants. These are chiefly benzene, toluene and benozpyrine etc. discharged by automobiles and industries.
These come from industries, refrigeration, air conditioning, sprays etc.
There are several toxicants other than heavy metals. These include PAN, acetic acid, chloroform, methylchloride etc.
9. Metallic Pollutants:
These mainly include Al, Be, Pb, Cd, Ni, As, Sn, V, Ti, Mn, Hg, Fe etc. These are present in air as solid particles or liquid droplets or gases. Mostly metallurgical processes and automobiles produce these.
These include SOx, NOx, PAN, PBzN, peracetic acid, acetyl peroxide etc. These are released mostly by automobiles.
These are formed in sunlight from hydrocarbons and NOx. These form acid droplets with other gases.
Essay # 3. Factors Affecting Air Pollution:
The factor which affect air pollution are as follows:
(i) Meteorological Characteristics:
Meteorological parameters like wind direction and magnitude, atmospheric lapse rates and relative humidity, etc. of a locality will influence the air pollution. Wind velocity and direction will carry the pollutant by advection (pollution carried by downwind velocity). The ground level concentrations are mostly depended on wind direction and magnitude and lapse rates. The mixing depth for the dispersion of pollutants is depended on lapse rates. The temperature variation with height will make the pollutants to move less or more speed.
Relative humidity is another important parameter which will influence temperature variations in a region. If temperature is more near the earth’s surface, water vapour will be more in the atmosphere (particularly in troposphere). Hence, relative humidity is more. It absorbs earth’s re-radiation and hold it in the troposphere (up to 2 km above the earth’s surface). These changes will influence the dispersion of pollutants in the atmosphere.
(ii) Topographical Features:
The dispersion of pollutants are affected by the unevenness of the land forms and barriers (obstacles) like mountains, etc. Topographical features will be advantageous and disadvantageous depending on the occasion and local conditions.
(iii) Characteristics of Pollutants:
The seriousness of air pollution problems are depended on the type and size of pollutants, either solid, liquid or gas. It may be also due to energy, either noise, or heat or radioactive, or combination of above. The interaction of pollutants in the atmosphere may reduce or increase the level of pollution, which is depended upon the characteristics of the pollutants.
(iv) Mode of Release of Pollutants:
The effect of air pollution is depended upon the way of entry of pollutants and also rate of release of pollutants. They may be released intermittently or continuous or cyclic or from a single source or from multiple sources or point and non-point sources. The dispersion of pollutants is also depended upon the mode of release of pollutants into the atmosphere.
Meteorological Factors Influencing Air Pollution:
The degree to which air pollutants discharged from various sources may concentrate in a particular area depends largely on meteorological conditions. Thus even though the total discharge of contaminants into the atmosphere in a given area remains constant from day to day, the degree of air pollution may vary widely because of differences in meteorological conditions. The important meteorological parameters that influence air pollution can be classified as primary parameters and secondary parameters.
Primary parameters are:
(1) Wind direction and speed
(2) Temperature
(3) Atmospheric stability
(4) Mixing height.
Secondary parameters are:
(1) Precipitation
(2) Humidity
(3) Solar radiation
(4) Visibility.
The meteorological parameters vary widely with latitude, season and topography. Further just as weather affects the severity of air pollution, air pollution, may in turn, affect weather conditions. Air pollution may influence the weather in several ways. Visibility may be reduced, fog frequency and duration may be increased and the incoming solar radiation may be decreased, particularly in the ultraviolet end of the spectrum.
Asbestos is the only naturally occurring fibrous mineral. This non-metallic substance possesses a unique property of resisting heat transfer even at high temperatures.
Essay # 4. Causes of Air Pollution:
Air pollution is caused by natural as well as man-made factors, on this basis the sources, polluting the air can be studied under two types:
(i) Natural sources
(ii) Human activities (Manmade sources)
(i) Natural sources:
Air is polluted by natural climatic activities, like cyclone, high velocity winds etc., which make the atmosphere full of dust and makes the visibility almost nil and it becomes difficult to inhale this dusty air. Dust cause many types of allergies and mainly affects ear, nose, throat and eyes.
Air pollution is also caused, due to flying ashes from volcanoes. It becomes difficult for asthmatic patients during the changing seasons.
(ii) Human activities (Man made sources):
This type of air pollution is caused due to various human activities.
These are as following:
(a) Combustion:
The process of combustion is one of the main reasons of air pollution. During burning of the fuel many types of gases, soot and small particles are produced. These, mix with the air and pollute it. The Suspended Particulate Matter (SPM), is quite harmful for our respiratory system and eyes.
The following combustion process cause air pollution:
i. Combustion in domestic activities:
Fire is produced by the combustion of various fuels like, coal, coke, wood, kerosene, etc. This combustion produces gases like carbon monoxide (CO), carbon dioxide (CO2), sulphur dioxide (SO2) etc., and mix with the air, in which we breathe, the oxygen needed in combustion of these fuels is also taken from the surroundings. In our country in most of the villages and smaller towns, forest wood, cow-dung, dry farm waste, dry grass and leaves are used as fuels these fuels produce more harmful gases as well as SPM.
ii. Combustion in thermal power stations:
To obtain heat, for producing electricity tons of coal and lignite are burnt. This produces great quantity of SO2, soot, fly ash, etc., which are just thrown into the air.
iii. Combustion in vehicles:
All the means of transport, bus, train, aeroplanes, scooter, car, run on the combustion engines, using petro, diesel as fuel. After combustion, the vacuum pipe of the vehicle evolves smoke and soot containing gases like nitrogen dioxide and peroxide, carbon mono and di-oxide and carbon particles as SPM. If there is lead in the petrol, it is a big pollutant itself.
With the increase in pollution, urbanization and consumerism, number of petrol and diesel driven vehicles is increasing every day the air pollution level of metro cities is given everyday in the news, which is mostly above the-safe limits.
iv. Combustion for disposal of garbage:
It is very common practice to burn, all types of garbage, expelled from, house, hotels, hospitals, gardens, farms etc. But all type of bio-degrad- able or non-biodegradable material produce, smoke and soot polluting the air.
v. Combustion in small and large scale industries:
In most of the industries, bit furances are installed for melting, fusion, evaporation etc., and tons of fuel like, coal, wood, is burnt, the smoke and SPM pollute the air.
(b) The crackers:
It is very common practice to burn crackers for entertainment, many shows are organized, on weddings and Diwali festival and a large amount of solid waste in the form of colloidal particles also get mixed in the air, we breathe. This increase SPM level in the air.
(c) Nuclear explosions:
The nuclear explosion in an area, pollute the air of a large area at once, inducing many hazardous chemicals, dust particles, in excess,
(d) The waste products coming out of the chimneys of industries:
The second, main, human made factor of air pollution is industrialization. For the all-round development of a country, industrialization is must but in absence of the proper treatment plants, all the harmful gases, hydrocarbon wastes and other hazardous chemicals are emitted out, in the atmosphere, polluting it.
Bhopal gas tragedy is worst accident ever happened in the country due to air pollution. The killing gas methyl isocyanide, was emitted out from union carbide fertilizer factory on the night of 2nd and 3rd Dec. 1984, which killed thousands of people and left many others handicapped.
(e) Through agricultural activities:
This is also a prominent factor of air pollution. To increase the yield, excess of harmful chemicals are sprayed on the crops, in the form of liquids and powders, these chemicals travel a large distance with air, polluting it. While processing the crops, the small particles in the form of saw dust etc., are also induced in the air.
(f) Due to many appliances, gadgets etc.:
In the devices like fridge, air conditioners etc., chloroflouro carbons (CFC) are used. These are the chemicals which diminish the protective ozone layer.
(g) Through smokers:
The people who smoke, cigarettes, bidi, cigar etc., pollute the air with smoke. This harms the non-smokers also as they inhale the smoke.
(h) Through cosmetics:
Most of the synthetic perfumes, deodorants, room fresheners are prepared, using the medium of solvents like ether, alcohol etc., when these sprays are used, they get mixed with the air.
(i) Use of solvents:
In the polish of floor, furniture, etc., and spray painting of vehicles, gudgets, many organic solvents are used, which evaporate at very low temperature. These are low boiling hydrocarbons. These harmful chemicals mix into the air, when used.
(j) Other sources:
Dead animals, rotten garbage, murshy gutter, tanneries, distrillaries, produce gases having very foul smell, this pollution can be felt in everyday life in our country.
Essay # 5. Effects of Air Pollution:
Substantial evidence has accumulated that air pollution affects the health of human beings and animals, damages vegetation, soils and deteriorates materials, affects climate, reduces visibility and solar radiation, impairs production processes, contributes to safety hazards, and generally interferes with the enjoyment of life and property.
Although some of these effects are specific and measurable, such as damages to vegetation and material and reduced visibility- most are difficult to measure, such as health effects on human beings and animals and interference with comfortable living.
Each of the effects listed above has been the subject of considerable attention, and a number of comprehensive reviews of the effects of air pollution have been written. In this article we shall present a brief summary of some of the most important established effects of air pollution.
i. Effects of Air Pollution on Atmospheric Properties:
Air pollutants affect atmospheric properties in the following ways:
1. Visibility reduction
2. Fog formation and precipitation
3. Solar radiation reduction
4. Temperature and wind distribution alteration
These effects are primarily associated with the urban atmosphere. In addition, there is much current interest in possible effects of air pollutants, mainly carbon dioxide and particles, on the atmosphere as a whole.
In addition to reducing visibility, air pollution affects urban climates with respect to increased fog formation and reduced solar radiation. The frequency of fog formation has been observed to be higher in cities than in the country in spite of the fact that air temperatures tend to be higher and relative humidities tend to be lower in cities as opposed to the country. The explanation for this observation lies in the mechanism of fog formation.
Scattering and absorption of both solar and infrared radiation, as well as emission of radiation, occur within the polluted layer. The net effect of these radiative processes during the night is a marked cooling of the pointed layer.
Studies in London, for example, have shown that the average duration of bright sunshine in central London (in hours per day) is discernibly less than in the surrounding countryside. In general, the decrease in direct solar radiation due to a polluted layer amounts to 10 to 20 percent.
ii. Effects of Air Pollution of Materials:
Air pollutants can affect materials by soiling or chemical deterioration. High smoke and particulate levels are associated with soiling of clothing and structures, and acid or alkaline particles, especially those containing sulfur, corrode materials, such as paint, masonry, electrical contacts, and textiles. Ozone is particularly effective in deteriorating rubber.
iii. Effects of Air Pollution on Vegetation:
Pollutants which are known phytotoxicants (substances harmful to vegetation) are sulfur dioxide, peroxyacetyl nitrate (an oxidation product in photochemical smog) and ethylene. Of somewhat lesser severity are chlorine, hydrogen chloride, ammonia, and mercury. In general, the gaseous pollutants enter the plant with air through the stomata in the course of the stomatal respiration of the plant.
Once in the leaf of the plant, pollutants destroy chlorophyll and disrupt photosynthesis. Damage can range from a reduction in growth rate to complete death of the plant. Symptoms of damage are usually manifested in the leaf, and the particular symptoms often provide the evidence for the responsible pollutant.
iv. Effects of Air Pollutants on Human Health:
We now come to the most controversial and probably the most important effect of air pollution, that is on human health. First we consider the mechanisms by which pollutants can effect the human body. We then discuss the type of evidence available on the effects of long-term exposure to pollutant levels characteristic of urban areas.
Pollutants enter the body through the respiratory system, which can be divided into the upper respiratory system, consisting of the nasal cavity and the trachea, and the lower respiratory system, consisting of the bronchial tubes and the lungs. At the entrance to the lungs, the trachea divides into two bronchial trees which consist of a series of branches of successively smaller diameter.
The entire bronchial tree consists of over 20 generations of bifurcations, ending in bronchioles of diameters of about 0.05 cm. At the end of the bronchioles are large collections of tiny sacs called alveoli. It is across the alveolar membranes that oxygen diffuses in the opposite direction. Although an individual alveolus has a diameter of only about 0.02 cm. there are several hundred million alveoli in the entire lung, providing a total surface area for gas transport of roughly 50 m2.
The respiratory system has several levels of defense against invasion by foreign material. Large particles are filtered from the airstream by hairs in the nasal passage and are trapped by the mucus layer lining the nasal cavity and the trachea.
These large particles are unable to negotiate the sharp bends in the nasal passage, and because of their inertia, impinge on the wall of the cavity as the air rushes down toward the lung. In addition, particles may also be scavenged by fine hair-like cilia which line the walls of the entire respiratory system. These cilia continually move mucus and trapped material to the throat where they are removed by swallowing. Most particles of sizes exceeding 0.5 to 5 pm are effectively removed in the upper respiratory system.
Particles of radii less than a few micrometers generally pass through the upper respiratory system, escaping entrapment. Some of the larger of these particles (about 1 pm in size) are deposited on the bronchial walls immediately behind bifurcations in the bronchial tree. The mechanism for this deposition is believed to be inertial impaction which results from the swirling air motions caused by the bifurcation.
Very small particles (radii <0.1 μm) are strongly influenced by Brownian motion (rapid, irregular movement due to collisions of the particle with air molecules). As a result, these particles have a high probability of striking the bronchial walls somewhere in the bronchial tree.
There is effectively a “window” in the size range 0.1 to 1 μm, where the particles are too large to be influenced by Brownian motion but are too small to be trapped in the upper portion of the lung. Particles in this size range are able to penetrate deep into the lung.
For gases, the solubility governs what proportion is absorbed in the upper airway and what proportion reaches the terminal air sacs of the lungs. For example, SO2 is quite soluble and consequently, is absorbed early in the airway, leading to airway resistance (swelling) and stimulated mucus secretion.
On the other hand, CO, NO2 and O3 are relatively insoluble and are able to penetrate deep into the lung to the air sacs. Nitrogen dioxide and ozone cause pulmonary edema (swelling) which inhibits gas transfer to the blood. Carbon monoxide is transported from the air sacs to the blood and combines -with hemoglobin as oxygen does.
It is important to note that more than one pollutant may induce the same effect. For example, sulfur dioxide and formaldehyde both produce irritation and increased airway resistance in the upper respiratory tract, and both CO and NO2 interfere with oxygen transport by hemoglobin. Several pollutants usually are present at the same time, and as a result, observed effects may actually be attributable to the combined action of more than one pollutant.
A good example of this is the case of SO2 and particulate matter. Health effects become far more serious when both are present then if either occurs separately. A possible explanation for this effect is that SO2 becomes absorbed on the surface of very small particles and is carried by the particles deep into the lung.
The effects of carbon monoxide exposure are reflected in the oxygen-carrying capacity of the blood. In normal functioning, hemoglobin molecules in the red blood cells carry oxygen, which is exchanged for carbon dioxide in the capillaries connecting arteries and veins. Carbon monoxide is relatively insoluble and easily reaches the alveoli along with oxygen.
The carbon monoxide diffuses through the alveolar walls and competes with oxygen for one of the four iron sites in the hemoglobin molecule. The affinity of the iron site for CO is about 210 times greater than for O2, so that this competition is extremely effective. When a hemoglobin molecule acquires a CO molecule it is called carboxyhemoglobin (abbreviated COHb).
The presence of carboxyhemoglobin decreases the overall capacity of the blood to carry oxygen to the cells. In addition, the presence of CO on one of the iron sites of a hemoglobin molecule not only removes that site as a potential carrier of an O2 molecule but also causes the other iron sites of the molecule to hold more tightly onto the O2 molecules they are carrying. The formation of COHb is a reversible process, with a half-life for dissociation after exposure of about 2 to 4 hr for low concentrations.
The major source of CO in urban areas is automobile exhaust. Levels typical of urban areas range from 5 to 100 ppm. It appears that the most serious danger associated with CO is the exposure of drivers on heavily congested highly congested highways to CO levels of the order of 100 ppm.
It has been found experimentally that relatively low COHb levels can affect the ability to estimate time intervals, can delay reaction times, and reduce visual sensitivity in the dark. The supposition that CO leads to increased incidences of traffic accidents, by virtue of effects such as these, is indeed a compelling one.
Sulfur dioxide is highly soluble and consequently is absorbed in the moist passages of the upper respiratory system Exposure to SO2 levels of the order of 1 ppm leads to constriction of the airways in the respiratory tract.
High SO2 levels are often associated with high particulate concentrations. The fact that a three- to fourfold increase in the irritant response to SO2 is observed in the presence of particulate matter is presumably attributable to the ability of the aerosol particles to transport SO2 deep into the lung.
There is no available evidence supporting the proposition that nitric oxide (NO) is a health hazard at levels found in urban air. Nitrogen dioxide (NO2), on the other hand, is transformed in the lungs to nitrosamines, some of which may be carcinogenic. In addition, NO2 may be transferred to the blood to form a compound called methemoglobin. Nitrogen dioxide is known to irritate the alveoli, leading to symptoms resembling emphysema upon long-term exposure to concentrations of the order of 1 ppm.
The term photochemical oxidants refers to the secondary pollutants formed in photochemical smog from reactions involving hydrocarbons and oxides of nitrogen. The principal ingredient in this category is ozone, with smaller amounts of oxygen- containing hydrocarbon compounds. The effect of ozone on pulmonary function is still not thoroughly understood.
In general, ozone of about 1 ppm produces a narrowing of the airways deep in the lung, resulting in increased airway resistance. The effects of long-term exposure to ozone at levels typical of urban air (about 0.1 to 0.2 ppm) have not been established.
Experiments with animals have exhibited irreversible changes in pulmonary function after long-term exposure to levels of 1 ppm. A topic of current speculation is that exposure to low levels of ozone accelerates the aging of lung tissue by the oxidation of certain compounds in proteins.
A widespread effect of photochemical smog is eye irritation. The precise mechanism by which certain compounds cause irritation of eyes is not known; in fact, it appears that the compounds responsible for eye irritation in smog may not all have been identified. Those that are known to be irritants and that have been detected in photochemical smog are formaldehyde (HCHO), acrolein (CH2CHCHO), and members of the family of peroxyacyl nitrates, two of which are-

The mechanisms of lead poisoning are complex. In short, lead inhibits several steps in the formation of hemoglobin. Depending on the mode of entry into the body, up to 60 percent of the total lead ingested can be permanently retained by the body.
Over the years, concentrations of air pollutants in a particular area have, on occasion, reached excessively high levels for periods of several hours to several days. The result has been a number of so-called air pollution episodes. During an episode, the person most likely to be seriously injured is one who is either elderly or is in questionable health, perhaps already suffering from a respiratory disease.
Disease of the respiratory system are generally correlated with air pollution. There are two types of reactions to air pollutants by the respiratory system. The first is acute reaction, such as irritative bronchitis, and the second is chronic reaction, such as chronic bronchitis and pulmonary emphysema.
Bronchitis refers to a condition of inflammation of the bronchial tree. The inflammation is accompanied by increased mucus production and a cough. Airway resistance is increased because of the presence of the thickened mucus layer. Acute bronchitis is generally a short-lasting disease, caused by a virus or foreign material in the lung.
Chronic bronchitis, on the other hand, is a sustained inflammation of the bronchial system, leading to an increase in the volume of mucoid bronchial secretion sufficient to cause expectoration. It is frequently accompanied by a cough and shortness of breath. The persistent inflammation leads to swelling of the terminal bronchi and increased airway resistance.
Emphysema is a condition in which the alveoli in the lung become uneven and over distended due to destruction of the alveolar walls. The disease is accompanied by shortness of breath, particularly following exercise.
The destruction of alveoli is progressive, resulting in an increased blood flow necessary to accomplish oxygen transfer and to a decreased ability to eliminate foreign bodies which reach the alveolar region. Emphysema has no known cure and is one of the fastest growing causes of death in the United States.
Ecological Effects of Air Pollution:
1. Chlorosis – The disappearance of chlorophyll is called chlorosis. It is caused by SO2 and fluorides present in the air.
2. Necrosis – The breakdown of cell is called necrosis. It is caused by SO2, nitrogen dioxide, ozone and fluorides.
3. Crop losses – Heavy loss of crop plants is caused by smog. Smog denotes a combination of smoke and fog. The important components of smog are ozone and PAN (Peroxy Acetyl Nitrate). They damage leafy vegetables, cereals, textile crops, ornamental plants, fruits and forest trees.
4. Nausea – H2S smells like rotten eggs and causes nausea.
5. Vomiting – SO2 causes vomiting.
6. Jaundice – Arsines induce RBC breakdown and jaundice,
7. Oxygen Carrying Capacity – CO reduces O2 carrying capacity of RBC by its permanent combination with hemoglobin.
8. Coughing – Coughing is induced by phosgenes (carbonyl chloride).
9. Headache – SO2 causes headache.
10. Cancer – Cancer is caused by air pollutants like ash, soot, smoke, chromium, nickel and radioactive elements.
11. Mutation – Radioactive elements produce mutation. Ozone produces chromosomal aberrations.
12. Cardiac Diseases – Cadmium causes high blood pressure and heart diseases.
13. Pneumonia – Pneumonia is caused by breathing in too much of manganese particles.
14. Acid Rains – The rain water having pH as low as 5.6 is called acid rain. This lowering of pH is due to the dissolution of acids in the rain water. Precipitation of oxides of sulphur and nitrogen with rain is termed as acid rain.
Acid rain is caused by air pollution. When atmospheric air contains sulphur dioxide (SO2) and oxides of nitrogen such as nitrous oxide (N2O) and nitric oxide (NO), they dissolve in rain water forming sulphuric acid and nitric acid. The rain water falls as acid rain.
The main source of oxides of sulphur and nitrogen is the burning of fossil fuels in power plants based on coal and oil contribute more than 60% of all sulphur oxides and 25 to 30% of nitrogen oxides in the atmosphere. Automobiles make a substantial contribution in large cities. Ozone is now recognized as a major factor in the formation of acid rain.
Acid rain affects both materials and organisms. It attacks building materials mainly sandstone, limestone, marble, steel and nickel. In plants, it leads to chlorosis (gradual yellowing in which the chlorophyll making mechanism is impeded) or depigmentation of leaves.
Acid rain increases the acidity of lakes and rivers. Vast tracts of forests and lakes in Europe and North America have been destroyed by acid rain. Acidity kills fish, bacteria and algae and the aquatic ecosystem collapses into sterility leaving a crystal clear but ultimately a dead lake.
Global Effects of Air Pollution:
i. Green House Effect-Global Warming and Rise in Sea Level:
The atmosphere that surrounds the earth plays a vital role in maintaining normal temperature on the earth’s surface. More than 99% of the atmosphere is made up by both nitrogen and oxide. Carbondioxide and other gases are present in smaller amounts.
Solar energy (light rays) from Sun travels in the form of electromagnetic waves as ultra violet, and other short wave radiation and also long wave radiation (Infra-red). The ultra violet rays are filtered by the ozone layer and the remaining moves towards the earth’s surface. The carbon dioxide present in the atmosphere strongly absorbs radiation in certain long wave infra-red portions of the spectrum.
The remaining solar radiation in the form of long wave and in the form of short wave are absorbed by the earth’s surface and converts to heat energy and emitted into space as long wave radiation. Water vapours and carbon dioxide are transparent to short wave radiation and they are nearly opaque to long wave radiation. A small portion of re-radiation long wave radiation passes to atmosphere.
The re-radiated long wave radiation passes to atmosphere. The re-radiated wave radiation from the earth’s surface again are absorbed by the carbondioxide and re- radiated back to earth’s surface. The latter helps to maintain a relatively warm condition between atmosphere and the ground and it thus helps to stabilize temporal variations in the earth’s surface temperature, which is most important for plants and animals.
Clouds and water vapour in the sky also serve to absorb and emit infra-red radiation and contribute to this heating effect. Thus, the atmosphere (mostly carbon dioxide) by absorbing re-radiated long wave radiation of earth acts as an insulation to keep the heat near the surface of the earth. This phenomenon is known as “Green House Effect”.
The word “Green House” is nothing but glass house which is constructed to grow tropical plants in cold areas of specific plants in tropical areas. The glass is used as roof cover in a greenhouse which is also transparent to short wave solar radiation and absorb the long wave radiation emitted from inside of the green house and block the re-radiated solar radiation, which maintain the heat sufficient for the plants growth.
This is similar to the process of solar short wave radiation reaching the earth’s surface and reflects back to the atmosphere from the earth’s surface in the form of long wave radiation. The carbon dioxide present in the atmosphere absorbs re-radiated long wave radiation and heat is maintained near the earth’s surface sufficient for living world.
Hence the latter process is called as “Green House Effect”. If more carbon dioxide is present (due to release of Carbon dioxide by fuel combustion) in the atmosphere more heat is retained near the earth’s surface.
It has been estimated that carbon dioxide will account for about half of the future temperature increases, while methane, nitrous oxide, ozone and chlorofluorocarbons will be responsible for the rest.
Greenhouse gases are produces both by industrial and biological processes, but while industrial emissions are fairly well documented. But, the knowledge of the production rates of greenhouse gases through biological processes and the factors regulating emissions is still inadequate. In the next 50 years, the increases in emissions of greenhouse gases to the atmosphere are estimated to result in global warming by 0.3 to 0.5 °C per decade.
The increase will not be evenly distributed, the largest increase are expected in northern latitudes during winter. These temperature changes will in turn affect the amount and temporal distribution of precipitation.
This may have drastic consequences for forestry and agriculture. Rising temperature will also lead to an increase in the levels of the world oceans due to a thermal expansion and the melting of the Arctic ice cap and glaciers. The increase over the next century is calculated to be 6 cm per decade. The effects on all coastal regions will be drastic.
The carbon dioxide level in the atmosphere is rapidly increasing due to burning of fossil fuels to obtain energy of transportation, electricity and heat generation. Since carbon dioxide absorbs infrared radiation, excess heat stays in the atmosphere and rises the mean temperature. If the earth continues to warm-up, all the glaciers and polar ice caps will begin to melt.
Many island countries will be submerged, coast line will be flooded due to the rise of sea level by few meter. It is estimated that one-third of the world’s population lives within 60 km of the coast line. In the next century, 25% of the world population may be affected by the rise in sea level due to global warming. In the last century, the sea level rose by 10 to 15 cm and by 2050 it is expected to rise further by 23 cm to 116 cm. By 2100 A .D. the rise in sea level may double to 56 cm to as much as 345 cm.
ii. Chlorofluorocarbons (CFCs) – Depletion of Ozone Layer:
The ozone layer of the stratosphere (a part of atmosphere) is present in the atmosphere at altitudes 10-60 km and it is most dense at 20-25 km. The average thickness of this layer is 3 millimeters.
Formation of Ozone Layer in the Atmosphere:
Ozone (O3), a gas composed of three oxygen atoms exists in a dynamic equilibrium in the stratosphere. The oxygen molecules which are produced by photosynthesis, diffused upwards by atmospheric circulation. It is decomposed by ultra violet short wave radiation. The sun emits light over a wide range of wave lengths including infrared visible and ultra violet.
The UV radiation which is having high energy damages the skin of man. Fortunately, the high energy UV radiation is removed in the upper atmosphere by a series of photochemical reactions involving molecular oxygen and ozone. Sun light striking an oxygen molecule breaks into two oxygen atoms.
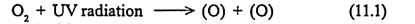
The atomic oxygen is highly reactive. Some of the oxygen atoms eventually combine with oxygen molecules and form ozone, which accumulate within the upper atmosphere and acts as filter by absorbing ultra violet radiation.

This reaction releases energy, but in the form of infrared rather than ultra violet radiation. If more of the UV radiation that reaches upper atmosphere can penetrate to the surface of the earth, which can cause burns and increases the chances of skin cancer. Thus the biologically harmful high energy ultra violet is converted to infrared radiation and helps to form ozone. The ozone then absorbs additional UV radiation and dissociate as oxygen and nascent oxygen, as given in the following equation.
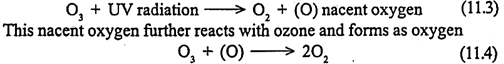
Thus UV radiation is removed by the formation and also the destruction of ozone. The ozone layer is crucial to life on earth since it acts as an ultra violet filter by absorbing much of the ultra violet from solar radiation before it reaches the earth’s surface. However this layer may be depleted by reactions involving a variety of compounds which reach the stratosphere.
The term “Acid rain” is defined as the rainfall which is more acidic (pH less than 5.6) than the normal natural water.
Generally the acidic characteristic of water is measured by pH, where normal range is 6.5 to 8.5. If the pH of rainfall is less than 6, is considered as acidic. The severe acidic nature of water occurs due to massive air pollution resulted by industrialisation. Hence to avoid acid rain, proper planning is necessary while locating the industries in a region or country.
Due to lack of planning and forecasting about acid rain, several developed countries are facing acid rain problems. Lakes and forests in wet Europe, Canada and U.S.A. and forests in West Germany are affecting due to acid rain. In India also acid rainfall has been reported in Mumbai (Bombay), Calcutta and Pune. The acid rain not only destructs the lakes and forests, but also it corrodes buildings, monuments, metallic surfaces etc.
Effects on Animals:
Air pollutants like arsenic, fluorine, lead, molybdenum and selenium will cause acute injury and death.
(i) Arsenic:
Arsenic poisoning causes sick in the animals due to the eating of grass with arsenic fall-out. Inflammation of the respiratory, gastro intestinal tract, destruction of red blood cells and kidney damage in cattle, horses and sheeps can occur. Acute poisoning symptoms are salivation, thirst, odour of garlic on the animal’s breath. Horses may have ulcers of the nose, difficult breathing.
(ii) Fluorine:
Cattle and sheep will be affected to fluorine poisoning (fluorosis). It occurs by ingestion of vegetation with fluoride containing matter.
(iii) Lead:
Acute lead poisoned animals give symptoms like foaming at the mouth and damage in the nervous system, etc.
Essay # 6. Dangers of Air Pollution:
Not everyone who is exposed to air pollutants will have health problems. Level, extent and duration of exposure, age, individual susceptibility and other factors play a significant role in determining whether or not someone will experience pollutants — related health problems. Every time we breathe polluted air, we risk inhaling particles and chemicals that can cause health problems for all of us.
The various hazards of polluted air include:
i. Respiratory diseases
ii. Headaches
iii. High blood pressure
iv. Heart problems
v. Kidney damage
vi. Neurological and brain damage
vii. Cancer
viii. Reproductive problems
ix. Other serious health problems.
Sensitive or Susceptible Population:
Much of the information about how pollution affects different group of people have come from studies done on urban particulate.
Children:
The saddest part of the story is that children are more affected by air pollution than the adults. In children, exchange of air between the lungs and the atmosphere is more than adults. As more air enters their lungs, so do proportionately more pollutants. Pollutants like lead are found at a level of one metre from the ground. Lead is probably the worst of the air-borne chemicals affecting mental health of children.
Susceptibility of children to air pollution can be due to several factors:
(a) Related to Lung Growth and Development:
i. Vulnerability of developing and growing airways and alveoli
ii. Immature host defense mechanisms
(b) Related to Time-Activity Patterns:
i. Time spent outdoors
ii. Increased ventilation with play and exercise
(c) Related to Chronic Disease:
i. High prevalence of asthma
ii. Rising prevalence of cystic fibrosis
(d) Related to Acute Disease:
i. High rates of acute respiratory infection.
ii. Several factors lead to increased exposure in children – compared to adults, they tend to spend more time outside; they engage in about three times the vigorous activity, and they breathe about 50% more air per pound of body weight.
Polluted air can impede lung function in children. It has been observed by studies that changes in annual average exposure to PM10 were associated with differences in annual lung function growth rates. Children who had moved to areas with lower PM10 showed increased growth in lung function and those that moved to areas with a higher PM10 showed decreased growth in lung function.
The Elderly:
Thousands of elderly people die prematurely each year from exposure to particulate pollution. Part of that is due to the fact that the elderly are more likely to have pre-existing lung and heart diseases. In addition, the elderly seem to be more affected than other age groups because important respiratory defense mechanisms are naturally lost in old age. Older individuals also tend to have more difficulty in clearing particles from their lungs.
As a result, pollutants do irritate the lungs for longer periods of time and can cause more damage. In addition, pollutants can compromise the immune system, increasing the susceptibility to bacterial or viral respiratory infections. This can lead to an increase in incidence of pneumonia and other complications among the elderly.
Individuals with Asthma, and other Respiratory Diseases:
Levels of pollutants which may not interfere with normal breathing may affect people with asthma in more profound ways, causing greater inflammation or constriction of airways. During an asthma attack, the muscles tighten around the airways, constricting the free exchange of air. The lining of the airways becomes inflamed and swollen. Children’s airways are narrower than those of adults, thus irritation that would produce only a slight response in an adult can result in significant obstruction in the airways of a young child. Older people with asthma experience higher mortality rates from asthma than other age groups.
Individuals with Cardiovascular Disease:
Cardiovascular diseases include many ailments such as hardening of the arteries, high blood pressure, angina pectoris, heart attacks and strokes. The exact toxicological mechanisms caused by air pollutants are not well understood. However, particulate matter causes respiratory symptoms, changes in lung function, alteration of muco-ciliary clearance and pulmonary inflammation that can lead to increased permeability of the lungs. This, in turn, can cause fluid to accumulate in the lungs. Mediators released during an inflammatory response could increase the risk of blood clot formation and strokes.
Smokers:
People who smoke have already compromised their lung functions. Exposure to high levels of particulate can exacerbate their condition, leading to chest pain, trouble breathing and other respiratory symptoms more quickly than in non-smokers.
Essay # 7. Control Measures to Check Air Pollution:
(i) Instead of using low grade and conventional fuels, use of non-conventional fuels like, Gobar gas, bio-gas compressed natural gas, LPG must be prepared and encouraged.
(ii) The vehicular pollution can be controlled by using good quality fuel, by keeping the engine fully effective, so that the fuel is totally burnt, by using unleaded petrol and by minimising the use of motored vehicles.
(iii) The industrial pollution can be reduced by following simple methods:
a. Instead of throwing industrial soot and smoke, directly into the air, it should be, first passed through electrostatic precipitators, where the suspended particulate matter (SPM) and other fine particles of hydrocarbons can be adsorbed and precipitated down, and the smoke becomes clean.
b. By adsorption on the surface of activated charcoal. The solid particles present in the industrial, gaseous waste are adsorbed when allowed to pass through a bed of activated charcoal. These are used in the chimney’s of industries.
c. The industrial gaseous waste is passed through scrubbers where high pressure water stream is lashed on the gases and they are dissolved in water, from where some useful substances can be recovered.
d. Air pollution can also be checked by converting the hazardous chemicals into the less harmful substances like carbon di-oxide, water etc.
(iv) The air quality can be improved and maintained by growing and developing green belts and forests.
Methods Used for Reducing Pollutants:
i. Pollutants can be separated from harmless gases because of difference in particle size. Particles which have size larger than 50 mm are separated by gravity settling tanks or porous filters. Smaller particles are separated by cyclone collector or electrostatic precipitator. Certain gases like ammonia are separated by dissolving in water.
ii. Pollutants can be converted to innocuous products before releasing them into the atmosphere.
Particulate air pollutants can be removed mainly by two types of devices – arresters and scrubbers.
Arresters are used to separate particulate matter from contaminated air and scrubbers are used to remove dust as well as gases by passing air through dry or wet packing material.
Some of the devices used to control particulate emission are as follows:
a. Mechanical Device:
Sudden change in the direction of the gas flowing causes the particles to separate out due to greater momentum. Small particulate matter from industrial emission with minimum moisture is separated by cyclonic separator.
b. Filters:
In this polluted air is passed through porous medium which collects fine particulate matter. The particulate matter from the gas gets trapped in the filter and clean air passes out.
c. Dry and Wet Scrubbers:
Both wet and dry scrubbers are used to separate dust. They are best suited for removal of gaseous pollutants. Wet scrubbers are used in chemical mining and metallurgical industries for the removal of SO2, NH3 and metal fumes.
d. Electrostatic Precipitator (ESP):
In this the gas containing pollutants like dust, aerosols and fumes are passed between two electrodes. As it passes through charged electrodes the particles precipitate on electrodes and settle down and clean gas passes out. This is effective in the removal of particulate pollutants.
Essay # 8. Prevention and Control of Air Pollution:
The control of air pollution is ultimately an engineering problem. The WHO (1968) in its publication.
“Research into Environmental Pollution” recommended the following procedures for the prevention and control of air pollution:
(i) Containment:
That is prevention of escape of toxic substances into the ambient air. Containment can be achieved by a variety of engineering methods such as enclosure, ventilation and air cleaning. A major contribution in its field is the development of “arrestors” for the removal of contaminants.
(ii) Replacement:
That is, replacing a technological process causing air pollution by a new process that does not. Increased use of electricity and natural gas in place of coal is an example of replacement.
(iii) Dilution:
Dilution is valid so long as it is within the self-cleaning capacity of the environment. For example, some air pollutants are readily removed by vegetation. The establishment of “green belts” between industrial areas is an attempt at dilution. The capacity for dilution is, however, limited and trouble occurs when the atmosphere is overburdened with pollutants.
(iv) Legislation:
Many countries have adopted legislation for control of air pollution. In India, there is a Smoke Nuisance Act effective in a few cities (i.e. Calcutta, Bombay, Ahmedabad and Kanpur). The Government of India have already drafted suitable legislation for control of air pollution.
(v) International Action:
To deal with air pollution on a world-wide scale, the WHO has established an international network of laboratories for the monitoring and study of air pollution. The network consists of two international centers at London and Washington, three centers at Moscow, Nagpur, and Tokyo and 20 laboratories in various parts of the world. These centers will issue warnings of air pollution where and when necessary.