Are you looking for an essay on the ‘Cell Surface Receptors and Its Types’? Find paragraphs, long and short essays on the ‘Cell Surface Receptors and Its Types’ especially written for school and college students.
Essay # 1. Cell Surface Receptors:
Cell surface proteins that bind signaling molecules external to the cell with high affinity and convert this extracellular event into one or more intracellular signals that in turn alter the behavior of the target cell. Cell surface receptors, unlike enzymes, do not chemically alter their ligands. They are glycoproteins that are embedded or otherwise attached to the plasma membrane and have a binding site for specific ligands (cytokines, hormones, growth factors, neurotransmitters, adhesion molecules, etc.) exposed to the extracellular environment.
Ligand binding to a cell surface receptor generally leads to a biological signal that is propagated from the receptor towards the cell interior, resulting in a cellular response such as proliferation, differentiation, apoptosis, degranulation, etc. Cell surface receptors transduce ligand signals by a variety of mechanisms such as receptor clustering, activation of a hidden enzymatic activity, opening of ion channels, etc.
Activation of all cell surface receptors leads directly or indirectly to the changes in protein phosphorylation through the activation of protein kinases or protein phosphatases. Animal cells contain two types of protein kinases- those that add phosphate to the hydroxyl group on tyrosine residues and those that add phosphate to the hydroxyl group on serine or threonine (or both) residues. Phosphatases, which remove phosphate groups, can act in concert with kinases to switch the function of various proteins on or off. The human genome encodes about 500 protein kinases and 100 different phosphatases. In some signaling pathways, the receptor itself possesses intrinsic kinase or phosphatase activity; in other pathways, the receptor interacts with cytosolic or membrane-associated kinases.
Essay # 2. Types of Cell Surface Receptors:
i. Steroid Receptors:
Steroids are small hydrophobic molecules that can freely diffuse across the plasma membrane, through the cytosol, and into the nucleus. Steroid receptors are dimers of zinc-finger proteins located within the nucleus (except for the glucocorticoid receptor which resides in the cytosol until it binds its ligand). Until their ligand finds them, some steroid receptors within the nucleus associate with histone deacetylases (HDACs), keeping gene expression repressed in those regions of the chromosome.
Some steroids that regulate gene expression include glucocorticoids (e.g., Cortisol), mineralocorticoids (e.g., aldosterone), sex hormones such as estradiol, progesterone and testosterone. The mechanism of action involves the steroid binds to its receptor and forms the complex, which releases the HDACs and recruits histone acetylases (HATs) relieving chromosome repression and binds to a specific DNA sequence called the Steroid Response Element (SRE) in the promoters of genes.
ii. Nitric Oxide (NO) Receptors:
NO diffuses freely across cell membranes and there are so many other molecules, with which it can interact, that it is quickly consumed close to where it is synthesized. Thus NO acts in a paracrine or even autocrine fashion i.e. affecting only cells near its point of synthesis. The signaling functions of NO begin with its binding to protein receptors in the cell. The binding sites can be either a metal ion in the protein or one of its sulfur atoms (e.g., on cysteine). In either case, binding triggers an allosteric change in the protein which, in turn, triggers the formation of a “second messenger” within the cell. The most common protein target for NO seems to be guanylyl cyclase, the enzyme that generates the second messenger i.e., cyclic GMP (cGMP).
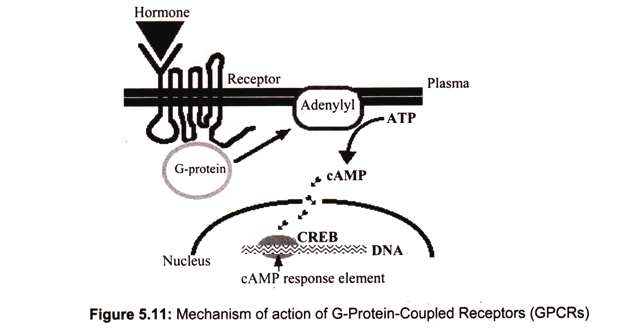
iii. G-Protein-Coupled Receptors (GPCRs):
G-protein-coupled receptors are transmembrane proteins that wind seven times back and forth through the plasma membrane. Their ligand-binding site is exposed outside the surface of the cell and their effector site extends into the cytosol. Some of the many ligands that alter gene expression by binding GPCRs include protein and peptide hormones such as thyroid- stimulating hormone (TSH) and ACTH and serotonin.
The mechanism of action involves the ligand binds to a site on the extracellular portion of the receptor. Binding of the ligand to the receptor activates a G protein associated with the cytoplasmic C-terminal. This initiates the production of a “second messenger”. The most common of these are cyclic AMP, (cAMP) which is produced by adenylyl cyclase from ATP, and inositol 1,4,5-trisphosphate (IP3).
The second messenger, in turn, initiates a series of intracellular events such as phosphorylation and activation of enzymes responsible for release of Ca2+ into the cytosol from stores within the endoplasmic reticulum. In the case of cAMP, these enzymatic changes activate the transcription factor CREB (cAMP response element binding protein). Here it binds to its response element 5′ TGACGTCA 3′ in the promoters of genes that are able to respond to the ligand, activated CREB turns on gene transcription and the cell begins to produce the appropriate gene products in response (Fig. 5.11).
A cell must also be able to stop responding to a signal, i.e., to turn off GPCRs. When activated, the Ga subunit of the G protein swaps GDP for GTP. However, the Ga subunit is a GTPase and quickly converts GTP back into GDP restoring the inactive state of the receptor. The receptor itself is phosphorylated by a kinase, which not only reduces the ability of the receptor to respond to its ligand but recruits a protein, β-arrestin, which further desensitizes the receptor, and triggers the breakdown of the second messengers of the GPCRs such as cAMP for some GPCRs.
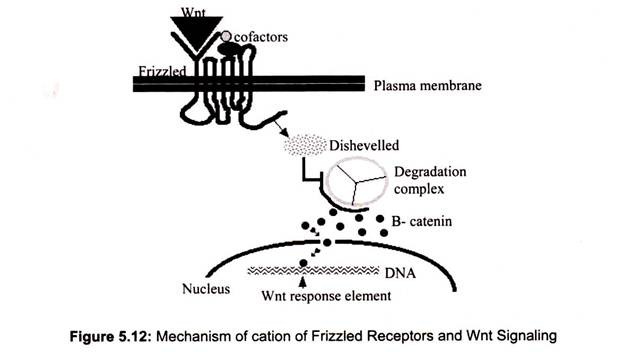
iv. Frizzled Receptors and Wnt Signaling:
Frizzled receptors, like GPCRs, are transmembrane proteins, their ligand-binding site is exposed outside the surface of the cell and their effector site extends into the cytosol. The ligands of the receptors are Wnt proteins. The name derived from two of the first discovered, proteins encoded by wingless (wg) in Drosophila and its homolog Int-1 in mice.
The binding of a Wnt ligand to Frizzled activates Frizzled, which in turn, activates a cytosolic protein called Disheveled. Activated Disheveled inhibits the β-catenin degradation complex so β-catenin (β-catenin molecules connect actin filaments to the cadherins that make up adherens junctions that bind cells together) escapes destruction by proteasomes and is free to enter the nucleus where it binds to the promoters and/or enhancers of its target genes (Fig. 5.12).
Wnt-controlled gene expression plays many roles in embryonic development (e.g., a gradient from low at the future head to high at the future tail establishes the anterior-posterior axis throughout the metazoa), it guides regeneration as well as regulatory activities in the adult body.
v. The Notch Signaling Pathway:
This pathway differs from many of the other signaling pathways in that the ligands as well as their receptors are transmembrane proteins embedded in the plasma membrane of cells. Thus, signaling in this pathway requires direct cell-to-cell contact. Notch proteins are single -pass transmembrane glycoproteins. They are encoded by four genes in vertebrates. However, the first notch gene was discovered in Drosophila where its mutation produced notches in the wings.
The mechanism of action involves when a cell bearing the ligand comes in contact with a cell displaying the notch receptor, the external portion of notch is cleaved away from the cell surface and engulfed by the ligand-bearing cell by endocytosis. The internal portion of the notch receptor is cut away from the interior of the plasma membrane and travels into the nucleus where it activates transcription factors that turn the appropriate genes on (and off). Proper development of virtually all organs such as brain, pancreas, GI tract, heart, blood vessels, mammary glands etc., depends on notch signaling. Notch signaling appears to be a mechanism by which one cell informs an adjacent cell as to which path of differentiation to take (or not take). Defects in notch signaling have been implicated in some cancers, e.g. melanoma.
vi. Cytokine Receptors:
The cytokines form a family of relatively small, secreted proteins (generally containing about 160 amino acids) that control many aspects of growth and differentiation of specific types of cells. During pregnancy prolactin, for example, induces epithelial cells lining the immature ductules of the mammary gland to differentiate into the acinar cells which produce milk proteins and secrete them into the ducts. Another cytokine, interleukin 2 (IL-2), is essential for proliferation and functioning of the T cells of the immune system; its close relative IL-4 is essential for the formation of functional antibody-producing B cells.
Cytokine receptors are important class of cell-surface receptors, whose cytosolic domains are closely associated with a member of a family of cytosolic protein tyrosine kinases, the JAK kinases. The mechanisms by which cytokine receptors and receptor tyrosine kinases become activated by ligands are very similar, and there is considerable overlap in the intracellular signal-transduction pathways triggered by activation of receptors in both classes. Dozens of cytokine receptors have been discovered. Most of these fall into one or the other of two major families: Receptor Tyrosine Kinases (RTKs) and Receptors that trigger a JAK- STAT pathway.
a. Receptor Tyrosine Kinases (RTKs):
The receptors are transmembrane proteins that span the plasma membrane just once. Some ligands that trigger RTKs includes insulin, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), fibroblast growth factor (FGF) and macrophage colony-stimulating factor (M-CSF)
The mechanism of action of RTKs involves the binding of the ligand to two adjacent receptors forming an active dimer. This activated dimer is a tyrosine kinase; an enzyme that attaches phosphate groups to certain tyrosine (Tyr) residues — first on itself, then on other proteins converting them into an active state. Many of these (the human genome encode 90 different tyrosine kinases) in this way activate a cascade of expanding phosphorylations within the cytosol.
Some of these cytosolic tyrosine kinases act directly on gene transcription by entering the nucleus and transferring their phosphate to transcription factors thus activating them. Others act indirectly through the production of second messengers. In this case the RTKs, stop responding to a signal (off mechanism) by quickly engulfing and destroying the ligand-receptor complex by receptor-mediated endocytosis. For growth factor receptors, failure to do so could lead to uncontrolled mitosis or cancer.
b. JAK-STAT Pathways:
The receptor consists of two identical single-pass transmembrane proteins (homodimers) embedded in the plasma membrane. Each of their cytoplasmic ends binds a molecule of a Janus kinase (“JAK”). The ligands which trigger JAK-STAT pathway includes interferons, most of the interleukins (IL-2, IL-3, IL-4, etc.), Growth hormone, Erythropoietin (EPO), Thrombopoietin and Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF).
The mechanism of action involves the binding of the ligand that activates the JAK molecules which phosphorylate certain tyrosine (Tyr) residues on each other as well as on one or another of several STAT (“Signal Transducer and Activator of Transcription”) proteins. These, in turn, form dimers which enter the nucleus and bind to specific DNA sequences in the promoters of genes that begin the transcription. The JAK-STAT pathways are much shorter and simpler than the pathways triggered by RTKs and so the response of cells to these ligands tends to be much more rapid.
vii. Transforming Growth Factor-Beta (TGF-β) Receptors:
Here the receptor, single-pass transmembrane proteins that, when they bind to their ligand, become kinases that attach phosphate groups to serine and/or threonine residues of their target proteins. Ligands for these receptors include Transforming Growth Factor-beta, activins, Bone Morphogenic Proteins (BMPs) and Myostatin, an inhibitor of skeletal muscle growth.
The mechanism of action involves the binding of the ligand to the extracellular portion of the receptors, results in gaining kinases activity which phosphorylate one or more SMAD proteins in the cytosol. The SMAD proteins move into the nucleus where they form dimers with another SMAD protein designated SMAD4. These dimers bind to a DNA sequence (CAGAC) in the promoters of target genes and with the aid of other transcription factors they enhance, or repress gene the transcription.
viii. Tumor Necrosis Factor-Alpha (TNF-α) Receptors and the NF-κB Pathway:
TNF-α is synthesized and secreted by macrophages and other cells of the immune system. Receptor consists of trimmers of three identical cell-surface transmembrane proteins. The ligands consist of TNF-a and Lymphotoxin.
NF-κB resides in the cytosol bound to an inhibitor called IκB. Binding of ligand to the receptor triggers phosphorylation of IκB, IκB then becomes ubiquinated and destroyed by proteasomes. This liberates NF-κB so that it is now free to move into the nucleus where it acts as a transcription factor binding to the promoters and/or enhancers of more than 60 genes NF-κB got its name from its discovery as a transcription factor bound to the enhancer of the kappa light chain antibody gene. However, it also turns on the genes encoding IL-1 and other cytokines that promote inflammation.
ix. The T-Cell Receptor for Antigen (TCR):
T cells use a trans-membrane dimeric protein as a receptor for a particular combination of antigen fragment nestled in the cleft of a glycoprotein encoded by genes in the major histocompatibility complex.
Activation of the TCR causes a rise in intracellular Ca2+ which activates calcineurin, a phosphatase which removes phosphate from NF-AT (“Nuclear Factor of Activated T cells”). Dephosphorylated NF-AT enters the nucleus, and with the help of accessory transcription factors (designated AP-1), binds to the promoters of some 100 genes expressed in activated T cells. The immunosuppressant drugs tacrolimus and cyclosporine inhibit calcineurin thus reducing the threat of transplant rejection by T cells.