Are you looking for an essay on ‘Flow Cytometry’? Find paragraphs, long and short essays on ‘Flow Cytometry’ especially written for school and college students.
Essay # 1. Introduction to Flow Cytometry:
Flow cytometry is now a widely used method for analyzing expression of cell surface and intracellular molecules, characterizing and defining different cell types in heterogeneous cell populations, assessing the purity of isolated subpopulations, and analyzing cell size and volume. It allows simultaneous multi-parameter analysis of single cells. It is predominantly used to measure fluorescence intensity produced by fluorescent-labelled antibodies detecting proteins or ligands that bind to specific cell-associated molecules, such as DNA binding by propidium iodide.
One of the fundamentals of flow cytometry is the ability to measure the properties of individual particles. When a sample in solution is injected into a flow cytometer, the particles are randomly distributed in three-dimensional space. The sample must therefore be ordered into a stream of single particles that can be interrogated by the machine’s detection system. This process is managed by the fluidics system. Flow cytometry uses the principles of light scattering, light excitation, and emission of fluorochrome molecules to generate specific multi-parameter data from particles and cells in the size range of 0.5 μm to 40 μm diameter.
Essay # 2. Instrumentation of Flow Cytometer:
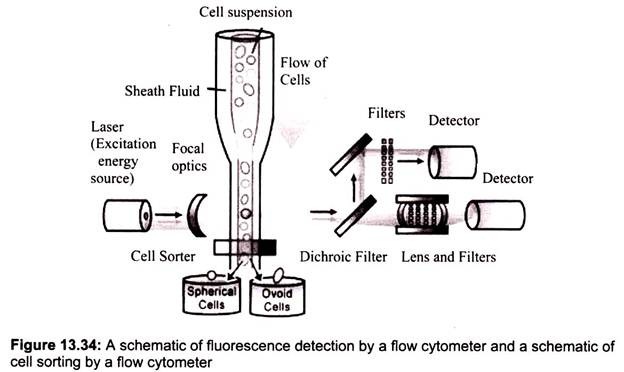
Flow cytometer, sometimes called a Fluorescence Activated Cell Sorter (FACS), has several key components (Fig. 13.34):
1. A light or excitation source, typically a laser that emits light at a particular wavelength;
2. A liquid flow that moves the suspended cells through the instrument and past the laser;
3. A detector, in this case a photomultiplier tube, which is able to measure the brief flashes of light emitted as cells flow past the laser beam.
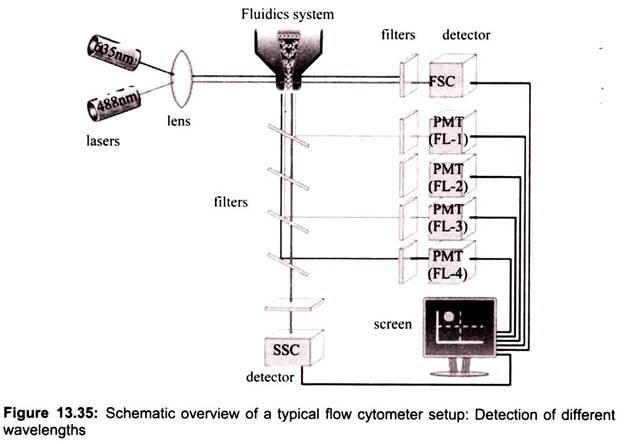
In a flow cytometer, single cells move past the excitation source and the light hitting the cells is either scattered or absorbed and then re-emitted (fluorescence). This scattered or re- emitted light is collected by the detector (Fig. 13.35).
(i) Light Scatter:
Scattered light is a consequence of a light beam making contact with a cell, resulting in either reflected or refracted light reaching the detector. The pattern of light scattering is dependent on cell size and shape, giving relative measures of these cellular characteristics as cells flow through the beam. This can be quite useful, as cells can be sorted on the basis of size or shape to different collection tubes using a technique called electrostatic deflection, which employs charged plates to change the path of the cell.
(ii) Fluorescence:
Fluorescence-based detection depends on the absorption of light by the cell and the subsequent re-emission of this light at a different frequency. Flow cytometers make use of this technology by employing filters to block the original light source from reaching the detector, while the fluorescence emission is allowed through for detection, which allows only a very low background of stray light to reach the detector. In flow cytometry experiments, fluorescence is often achieved by the deliberate labeling of a cellular component using a fluorescent marker, usually a type of dye. These dyes fluoresce only when light of the appropriate wavelength (specified by the frequency of the laser) hits them, causing the emission of secondary light at a different wavelength. Detection of the second wavelength is used as a measure of the presence of the dye on the cell and thus the component it is labeling. The most common fluorescent dyes are Texas-Red, fluorescein isothiocyanate (FITC) and phycoerythrin (PE).
Essay # 3. Applications of Flow Cytometry:
Flow cytometry is used in a variety of different fields including immunology, pathology and medicine, as well as in plant breeding.
A few of the most common applications are listed below:
a. DNA Content:
Fluorescence staining of DNA followed by flow analysis has been used to determine a cell’s DNA content. Stained cells with one copy of their genetic material (a haploid cell) will be half as bright as cells with two copies (a diploid cell). A cell varies between these states during the cell cycle and flow cytometry can be used to determine its position in the cell cycle based on its DNA content.
b. Evaluation of Cell-Surface Markers:
Immunologists frequently use flow cytometry to determine the types of markers and receptors on the surface of a cell. For these experiments, a fluorescent dye is attached to antibodies or receptor ligands. These cells can then be subjected to flow cytometry and the amount of the receptor on their surface detected as a level of fluorescence.
These experiments can be designed to incorporate more than one fluorescent marker at a time, giving the ability to detect multiple cell-surface markers simultaneously. Building upon the example of staining for particular markers or receptors, staining with more than one fluorescence dye allows researchers to determine whether there are populations of cells that contain multiple receptors. A specific example is the analysis of the markers on T-cells. T-cells are a type of immune cell, which have cell surface marker proteins known as CD4 and CD8.
In mammals there are T-cells that are CD4 positive and also the cells that are CD8 positive and some cells are positive for both markers. To determine the relative abundance of cells carrying the different markers, FITC-attached CD4 antibodies (normally termed FITC- conjugated CD4 antibodies) and Texas Red-conjugated CD8 antibodies could be incubated with T-cells. In flow cytometry analysis cells that were CD4 positive fluoresce green, while cells that were CD8 positive fluoresce red and cells that were positive for both markers give green and red light. Detection of the levels of each fluorescent color would give a measure of how many of each type of T-cell was present in the original mixture.
c. Cell Sorting:
Flow cytometry can be used to select and purify a specific subset of cells within a population. This is a popular application with researchers, since it allows the selection of cells expressing a particular receptor, in a phase of the cell cycle, or perhaps expressing a particular transgenic protein, followed by the culture of these cells as a pure population. Amazingly, a FACS can sort cells as fast as 15,000 cells/sec with very high purity (over 98%).