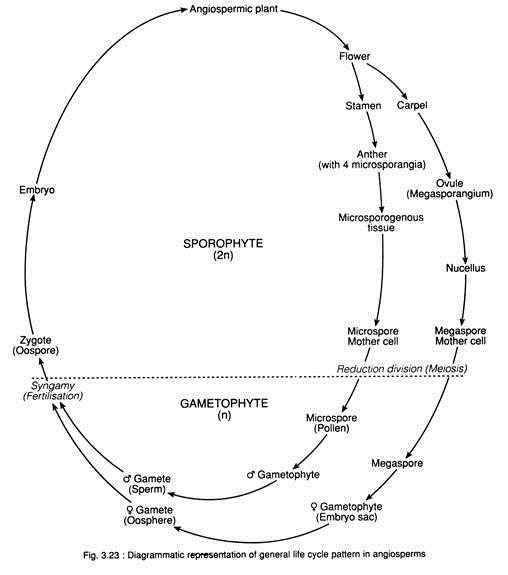
In this essay we will discuss about the process of fertilisation in angiosperms. After reading this essay you will learn about:- 1. Germination of Pollen Grains 2. Growth of Pollen Tube 3. Entry of Pollen Tube into the Ovule 4. Entry of Pollen Tube into the Embryo Sac 5. Release of Male Gametes from Pollen Tube 6. Fusion 7. Development of Embryo (i.e., Embryogeny) 8. Endosperm and Other Details.
Essay # 1. Germination of Pollen Grains:
During germination, pollen grain develops pollen tube. The pollen grain usually develops only one pollen tube (monosiphonous), e.g., Zea mays of Poaceae etc., but in some plants it develops more than one tube (polysiphonous). The polysiphonous condition is reported in the members of Malvaceae (Althea rosea develops 10 pollen tubes, Malva neglecta develops 14 pollen tubes) and Cucurbitaceae. Sometimes, the single pollen tube may be branched.
Essay # 2. Growth of Pollen Tube:
The pollen tube grows through the style and reaches the ovule.
Based on the internal condition of the style, which helps the movement of pollen tube, the styles are of the following three types:
1. Closed style:
Style consists of solid core of elongated cells with dense protoplasm, through which movement of the pollen tube takes place, e.g., Gossypium, Datura etc.
2. Open style:
Style is a hollow tube lined by special secretory cells. The pollen tube creeps through this tube, e.g., most members of monocotyledons, members of Papaveraceae.
3. Half closed style:
It is like the open type, but the canal is surrounded by a rudimentary transmitting tissue, e.g., members of Cactaceae.
Essay # 3. Entry of Pollen Tube into the Ovule:
The generative cell divides to form two male gametes, either within the pollen grain before germination or inside the pollen tube during gemination. After reaching the top of the ovary, pollen tube may enter through different routes.
According to the method of entry of pollen tube inside the ovary, it is of three types:
a. Porogamy:
When the pollen tube enters the ovule through the micropyle, the method is called porogamy. This process is very common.
b. Chalazogamy:
When the pollen tube is found to enter the ovule through chalaza, the method is called chalazogamy. It is found in Casuarina of Casuarinaceae, members of Betulaceae, Fagaceae etc.
c. Mesogamy:
When the pollen tube is found to enter the ovule through funiculus or the integument, the method is called mesogamy. e.g., Cucurbita of Cucurbitaceae etc.
Essay # 4. Entry of Pollen Tube into the Embryo Sac:
After the entry of pollen tube inside the ovary, it proceeds through the nucellus and reaches the embryo sac.
The pollen tube is then progressed inside, either:
1. between one synergid and the egg, or
2. between one synergid and the wall of embryo sac, or
3. directly into one synergid, thereby it is being destroyed.
Essay # 5. Release of Male Gametes from Pollen Tube:
After the entry of pollen tube inside the embryo sac, the gametes are released following either of the three processes:
1. Two openings are formed just behind the apex of the pollen tube and one gamete is discharged through each opening.
2. Bursting takes place at the apex of the pollen tube and the gametes are discharged, or
3. The apex of the pollen tube divides into two unequal short branches, of which the shorter one reaches the egg and the longer one towards the secondary nucleus. Later on, the apex of each branch bursts and the gametes are released.
Essay # 6. Fusion:
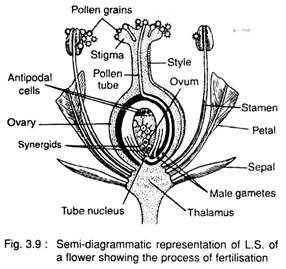
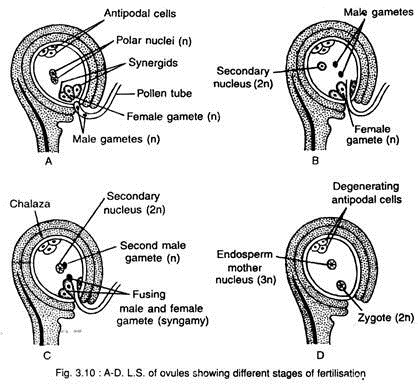
The fusion between male and female gametes is called syngamy and the fusion between male gamete and definitive nucleus i.e., secondary nucleus (formed by the fusion of two polar nuclei) is called triple fusion (Fig. 3.10A-D).
After releasing from the pollen tube, one male gamete (n) fuses with the egg (n) and forms the oospore or zygote (2n). This is called syngamy or true fertilisation. The other male gamete (n) fuses with the definitive nucleus i.e., secondary nucleus (2n) and forms a triploid nucleus (3n), called endosperm mother nucleus i.e., the first cell of the endosperm. This is called triple fusion.
Both the male gametes take part in fertilisation i.e., the fertilisation takes place twice. This is called double fertilisation. So the double fertilisation includes syngamy or true fertilisation and triple fusion. The double fertilisation was first observed by Nawaschin (1898) in species of Fritillaria and Lilium.
The oospore or zygote develops into embryo and the endosperm mother nucleus develops the endosperm. The endosperm is used for nutrition by the developing embryo.
The other components i.e., synergids and antipodal cells, gradually degenerate after fertilisation.
Essay # 7. Development of Embryo (i.e., Embryogeny):
During post-fertilisation, both the embryo and the endosperm develop simultaneously. After syngamy, the oospore takes rest for a period and then develops into an embryo. Both dicotyledonous and monocotyledonous plants do not show any difference in their early stages of development, but difference is visible in the latter stages.
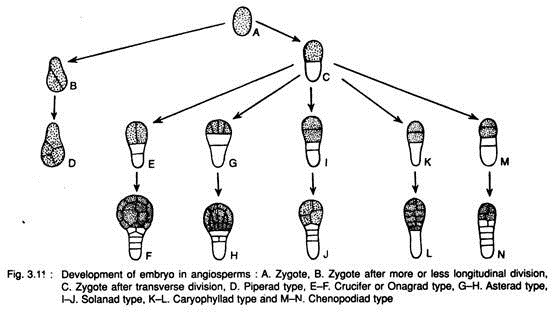
Johansen (1950) recognised six types of embryo development in angiosperms based on the patterns of differentiation of the terminal and basal cells.
These are:
1. Piperad type, e.g., Peperomia, Balanophora etc.
2. Crucifer or Onagrad type, e.g., commonly found in Brassicaceae (Cruciferae), Ranunculaceae, Onagraceae, Rutaceae etc.
3. Asterad type, e.g., members of Compositae, Polygonum of Polygonaceae etc.
4. Caryophyllad type, e.g., members of Caryophyllaceae, Saxifraga of Saxifragaceae etc.
5. Solanad type, e.g., members of Solanaceae, Linum of Linaceae, Papaver of Papaveraceae etc.
6. Chenopodiad type, e.g., members of Chenopodiaceae etc.
The above types of embryo development can be represented schematically (Fig. 3.11):
I. The first division of the oospore is longitudinal (Fig. 3.11B, D): Piperad type
This type occurs in Peperomia of Piperaceae, Scabiosa of Asteraceae and Balanophora of Balanophoraceae.
II. The first division of the oospore is transverse:
A. The division of the apical cell of the two celled proembryo is longitudinal:
1. The basal cell plays no part or only a minor part in the subsequent development of embryo (Fig. 3.11E, F). Crucifer or Onagrad type
This type occurs in Capsella of Brassicaceae, Veronica of Verbenaceae, Euphorbia of Euphorbiaceae, Mentha of Lamiaceae etc.
2. The basal as well as apical cells contribute to the development of embryo (Fig. 3.11G, H). Asterad type
This type occurs in almost all members of Compositae (Asteraceae), Polygonum of Polygonaceae, Oxalis of Oxalidaceae, Poa of Poaceae, Urtica of Urticaceae, Lamium of Lamiaceae etc.
B. The division of the apical cell of the two celled proembryo is transverse:
a. The basal cell plays no or only a minor part in the subsequent development of embryo.
1. The basal cell commonly forms 2 or more celled suspensor (Fig.3.11I, J) Solanad type
Physalis, Nicotiana, Datura and Hyocyamus of Solanaceae; Sherardia of Rubiaceae, Papaver of Papaveraceae, Hydnora of Hydno- raceae, Linum of Linaceae etc.
2. The basal cell undergoes no further division and suspensor (if present) is always developing from the terminal cell (Fig. 3.11 K, L). Caryophyllad type
This type is found in Sagina of Caryophyllaceae, Medicago of Fabaceae, Sagittaria of Alismataceae, Drosera of Droseraceae, Ruppia of Potamogetonaceae etc.
b. Both basal cell and apical cell contribute to the development of embryo (Fig. 3.11M, N). Chenopodiad type
This type occurs in Myosotis of Boraginaceae, Beta and Chenopodium of Chenopodiaceae and Polemonium of Polemoniaceae etc.
a. Typical dicotyledonous type (Crucifer type):
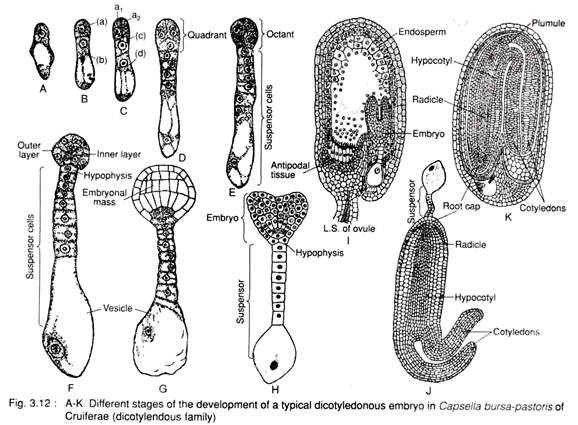
The typical dicotyledonous type of embryo is found in Capsella bursa-pastoris of Brassicaceae (Cruciferae). The ovule is campylotropous type, thus the embryo sac and the endosperm as well as embryo become horseshoe- shaped and the embryo looks upside down.
The oospore (Fig. 3.12A), first divides transversely into a large basal cell (b) and a small terminal cell (a), (Fig. 3.12B). The basal cell divides transversely and forms two cells, c and d, whereas the terminal cell (a) divides longitudinally to form two cells (a1 and a2). Thus, a 4- celled proembryo is formed (Fig. 3.12C).
Both the cells (c and d) of basal region, undergo repeated transverse division and form 5- 10 celled suspensor (Fig. 3.12D, E). The lowermost cell of the suspensor becomes swollen disproportionately and forms a vesicle, called the vesicular cell or the haustorial cell. The suspensor pushes the developing embryo into the endosperm and the basal cell functions as haustorium. The uppermost cell of the suspensor is called hypophysis (Fig. 3.12F).
Meanwhile, the two terminal cells further divide longitudinally at right angle to the first longitudinal division and form a quadrant i.e., 4 celled structure (Fig. 3.12D). The quadrant then undergoes transverse division and forms octant i.e., 8 celled structure (Fig. 3.12E).
The octant then divides periclinally (i.e., parallel to the peripheral surface) and forms outer and inner layers (Fig. 3.12F). The terminal octant mass with peripheral covering is called embryonal mass (Fig. 3.12G). The embryonal mass along with hypophysis i.e., the terminal cell of suspensor (h) divides further and forms a mass of tissue.
Ultimately the different sets of tissue form the different structures of a seed which are:
1. Four terminal cells of the octant differentiate to form plumule and two cotyledons.
2. Four basal cells of the octant form hypocotyl and the core of the radicle.
3. Hypophysis differentiates to form the cortex and the epidermis of the radicle as well as the root-cap.
The long suspensor pushes the embryo towards the middle of the embryo sac. Further development of embryo takes place at two different points of the lower tier of octant, thus forming a more or less cordate structure (Fig. 3.12H). With further development by cell division, the hypocotyl and cotyledon become horse shoe-shaped (Fig. 3.12I, J). This shape appears as per the shape of the embryo sac. During further maturation, the suspensor cells wither gradually.
This typical type of embryo development is called Crucifer type or Onagrad type.
The endosperm formed in the embryo sac is mostly consumed by the embryo. In mature seeds the remnant also disappears and thus the seed becomes exalbuminous (Fig. 3.12K).
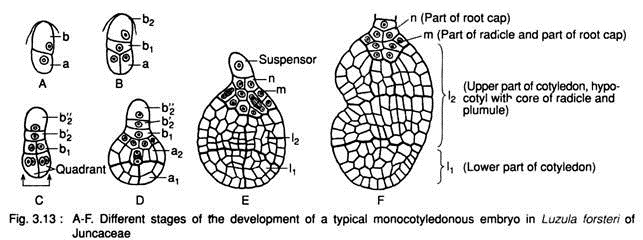
b. Typical monocotyledonous type:
It is found in Luzula forsteri of Juncaceae. The basic development of embryo up to octant stage is found to be similar in both dicotyledonous and monocotyledonous plants.
The zygote divides transversely into a basal cell (b) and a terminal cell (a) i.e., two-celled proembryo (Fig. 3.13A). The terminal cell (a) then divides longitudinally, while the basal cell (b) divides transversely forming two cells (b1and b2) (Fig. 3.13B). Thus 4-celled proembryo is formed.
Two terminal cells then divide longitudinally at right angle to the first one and develop a quadrant (Fig. 3.13C). A little later, at the basal region the cell (b,) divides longitudinally and the other cell (b2) divides transversely into b2” and b2” cells (Fig. 3.13C). The cells of b, further divide and give rise to a tissue m (Fig. 3.13D, E). The b2” cell acts as suspensor and the rest cells take part in the formation of embryo.
The quadrant of apical region undergoes divisions and forms a globose tissue. The tissue then undergoes periclinal divisions and forms outer and inner layers. The globose tissue of the terminal region becomes differentiated into two regions, the extreme terminal region (a1) and behind it is the a2 region (Fig. 3.13D). With further division both a, and a2 regions enlarge in volume and designated as I1 and l2 region, respectively (Fig. 3.13E).
Ultimately the different sets of tissue (Fig. 3.13F) form the different structures of a seed:
1. The I1 region gives rise to lower part of the cotyledon.
2. The l2 region gives rise to upper part of the cotyledon, the hypocotyl with core of radicle and later the plumule,
3. The cells of m becomes differentiated into upper part of the radicle and part of root cap,
4. The n develops the part of root cap,
5. The b2‘ develops the tip of root-cap, and
6. The b2” cell acts as suspensor.
Essay # 8. Endosperm:
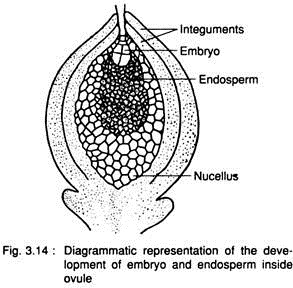
The endosperm plays a very important role, because it is the main source of food for the growing embryo (Fig. 3.14). In angiosperms, the endosperm forms after fertilisation and is triploid (3n) in nature as it is formed by triple fusion i.e., fusion of two polar nuclei (n + n) and further fusion with one of the male gametes (n) during double fertilisation.
But in gymnosperms, the endosperm forms before fertilisation and is haploid (n) in nature, which is formed by the repeated divisions of megaspore nucleus. Actually, it represents the female gametophyte.
Endosperm formation is suppressed in the families Orchidaceae, Podostemonaceae and Trapaceae. In those families double fertilisation takes place, but the triploid nucleus undergoes one or two divisions or degenerates immediately. Other angiosperms show three general types of endosperm development.
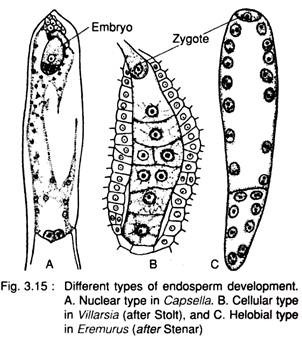
These are (Fig. 3.15):
1. Nuclear type,
2. Cellular type and
3. Helobial type.
1. Nuclear type:
The endosperm mother nucleus (3n) undergoes repeated divisions, but without wall-formation. During later stage of development the nuclei may remain free or wall-formation follows, e.g., species of Capsella, Mangifera, Calotropis, Asclepias, etc.
2. Cellular type:
The endosperm mother nucleus (3n) undergoes repeated divisions, accompanied by wall formation. Thus it becomes a multicellular structure, e.g., species of Peperomia, Villarsia, Adoxa and many other members of Boraginaceae, Annonaceae, Aristolochiaceae, Gentianaceae etc.
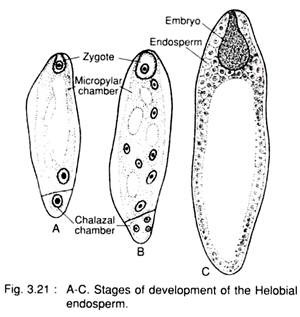
3. Helobial type:
It is an intermediate between nuclear and cellular type and is common in the order Helobiales (e.g., Vallisneria, Limnophyton, Eremurus etc.). A partition wall develops between the two nuclei results from the first division of the endosperm mother nucleus (3n) and thus a very big micropylar cell and a small chalazal cell are formed. The micropylar cell divides by free nuclear division, later followed by wall formation and the nucleus of the chalazal cell undergoes a few or no division at all.
The development of different types of endosperm can be described briefly as:
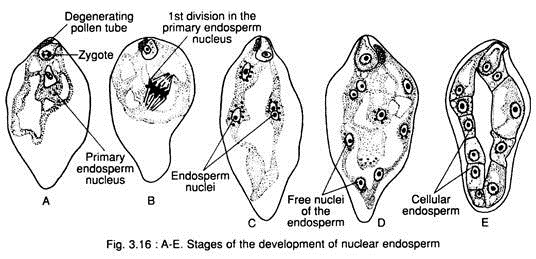
Nuclear type:
In this type, the first few divisions of the primary endosperm nucleus remain free in the cytoplasm of the embryo sac without forming any partition wall. The nuclei may remain free indefinitely (e.g., Acer pseudoplatanus, Limanthes douglasii etc.) or wall formation takes place subsequently (e.g., Arachis hypogea, Glycine max etc.), (Fig. 3.16A-E).
Initially, few divisions of the primary endosperm nucleus are synchronous, but later on, the divisions become irregular. Number of successive divisions normally depends on the size of the embryo sac.
The nuclei are pushed towards the periphery of the sac; as a result a vacuole is formed in the centre. Sometimes, some of the nuclei become aggregated towards both the chalazal and micropylar ends and the rest remains suspended in the periphery of cytoplasm, lining the wall of the embryo sac.
The nature of further growth and their behavior vary with plants.
It is generally of 4 types:
(a) Primary endosperm nucleus forms hundreds of free nuclei which remain along the periphery of the embryo sac and the wall-formation takes place at a very late stage, e.g., Mangifera, Citrus, Arachis, Malva etc.
(b) Wall-formation takes place only at 8- or 16-nuclear stage, e.g., Calotropis, Xeranthenum etc.
(c) Wall-formation does not take place and the nuclei remain free throughout, e.g., Cardiospermum, Lopezia, Tropaeolum, etc.
(d) Primary endosperm nucleus undergoes first or second division, but the nuclei degenerate — thus the further growth is ceased, e.g., Arachis hagenbackii.
All nuclei of endosperm are not of the same size. Commonly the chalazal nuclei are larger than those of the micropylar nuclei. But the micropylar nuclei are larger than chalazal nuclei in Fagraea.
Usually wall formation takes place centripetally i.e., from periphery towards the centre (e.g., Euphorbia, Bocconia etc.) or it may start at the micropylar end (e.g., Rumex, Polygonum, Phaseolus etc.).
But the extent of cell formation also varies:
(a) Entire endosperm may be cellular, e.g., Gagea stipitala, Allium fistulosum, Nyssa sylvatica etc., or
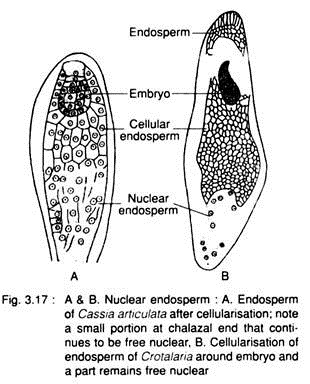
(b) Entire endosperm does not become cellular, e.g., Arachis, Cassia, Crotalaria (Fig. 3.17) etc.
Cocos nucifera is an unique example of nuclear endosperm. The watery liquid endosperm contains many free nuclei. It is known as liquid syncitium. Later on, cell formation takes place centripetally. The tissue thus formed is called coconut meal. The liquid endosperm contains growth-promoting coconut milk factor, used as nutrient medium in laboratory cultures.
Endosperm haustoria:
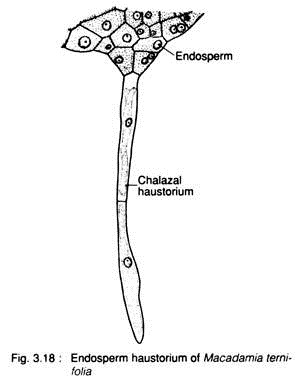
The endosperm haustoria absorb the nutrition from the nucellar tissue. In members of Cucurbitaceae, Leguminosae (Fabaceae) etc., the cell formation is restricted towards the micropylar end and nuclei at the chalazal end remain free. The coenocytic part of this chalazal end develops tubular haustorium, e.g., Macadamia ternifolia (Fig. 3.18).
Cellular type:
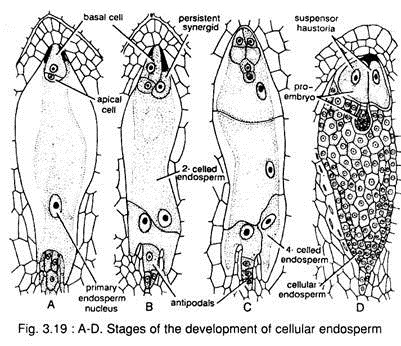
There is no free nuclear phase in this type, because the wall-formation starts with the first division of the primary endosperm nucleus (Fig. 3.19).
The primary endosperm nucleus may remain at the centre or at the chalazal end (e.g., Scrophularia etc.). The first division is usually transverse and divides the embryo sac into chalazal and micropylar chambers. But in some cases, it may be vertical or oblique.
Based on the pattern of wall-formation of a few subsequent divisions of primary endosperm nucleus, it is divided into 5 types:
(a) The first division of primary endosperm nucleus is vertical (i.e., longitudinal to the embryo sac) and the second one is also vertical, but at right angle to the first one — thus four elongated cells are formed. The subsequent wall-formations take place at the micropylar end only, e.g., Cetranthus, Adoxa etc.
(b) The first division of primary endosperm nucleus is transverse and then one or both the daughter cells divide vertically, e.g., Verbascum of Verbenaceae etc.
(c) The first division of primary endosperm nucleus is transverse and then one or both the daughter cells again divide transversely, e.g., members of Annonaceae and Palmae (Arecaceae).
(d) The first division of primary endosperm nucleus is oblique and the resultant two cells may be equal or unequal in size, e.g., Myosotis arvensis of Boraginaceae.
(e) The orientation of the first wall is indefinite, e.g., Gunnera, Senecio etc.
Endosperm haustoria:
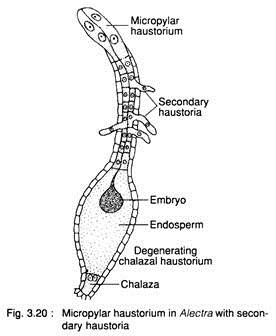
In this type, one or more cells become specialised and function as haustoria. The haustoria are formed at chalazal or micropylar or at both the ends. The haustoria penetrate into the nucellus and draw nutrition. Sometimes a few secondary haustoria are formed in addition to chalazal or micropylar haustoria, e.g., Alectra (Fig. 3.20).
Helobial type:
In this type, the first division of the primary endosperm nucleus is transverse, resulting into the formation of a large micropylar and small chalazal chamber (Fig. 3.21). Later on, the nuclear divisions continue in micropylar chamber, while the nucleus of the chalazal chamber remains undivided or undergoes a few divisions.
Simultaneously, wall-formation takes place in the micropylar chamber and it becomes multicellular, whereas wall formation does not take place in the chalazal chamber. The chalazal chamber contains one or a few disorganised nuclei.
Endosperm haustoria:
In this type, the haustoria normally develop from the micropylar tissue. They are unicellular, tube-like and penetrate the nucellar tissue at the chalazal end. It is the unique character of endosperm in the order Helobiae under monocotyledons.
Histological nature of endosperm:
The endosperm cells are usually isodiametric in shape and can store food materials. The nature of endosperm tissue varies from plant to plant. Generally, they are thin-walled and without pits. The cells of endosperm tissue are devoid of intercellular space. Endosperm may be oily in castor, starchy in rice etc. In cereals like rice etc., the outermost layer of endosperm is made of aleurone tissue (protein).
Fate of endosperm:
Depending on the presence or absence of endosperm in the mature seed, the seeds are classified into two types:
1. Endospermic seeds, and
2. Non-endospermic seeds
1. Endospermic seeds (albuminous seeds):
The seeds where the endosperm forms a permanent tissue which persists till the germination of seeds are called endospermic seeds. They are also called albuminous seeds, e.g., date palm, Phoenix syivestris and coconut, Cocos nucifera of Arecaceae (Palmae); wheat, Triticum aestivum and maize, Zea mays of Poaceae (Gramineae), etc.
2. Non-endospermic seeds (exalbuminous seeds):
The seeds where the endosperm becomes used up by the growing embryo and is no longer seen in mature seeds, are called non-endospermic seeds. They are also called exalbuminous seeds, e.g., pea, Pisum sativum and ground nut, Arachis hypogea and many others of Fabaceae; pumpkin, Cucurbita pepo and many others of Cucurbitaceae.
Morphological nature of endosperm:
In gymnosperms, the endosperm forms before fertilisation from the megaspore and is gametophyte in nature i.e., haploid. However, in angiosperms, it develops after fertilisation and is triploid. However, its morphological nature is a debatable question.
The considerations of the different workers regarding its nature are:
1. As gametophyte. Strasburger (1900), Coulter and Chamberlain (1911) considered the endosperm as gametophyte.
2. As sporophyte. Monnier (1980) considered the endosperm as sporophyte.
3. As an entirely new structure (i.e., Tissue sui generic). Brink and Copper (1940) stated that double fertilisation causes the entry of extra amount of chromosome, thereby it becomes more active in collecting food from the integument and nucellus which compensates the extremely reduced gametophyte of angiospermic plants.
Essay # 9. Formation of Fruit and Seed:
1. Formation of fruit:
Fruit is a mature ovary enclosing the seeds. The fruit wall develops from the ovarian wall (Table 3.1).
2. Formation of seed:
After double fertilisation, many changes takes place in ovule and finally the ovule becomes converted into seeds (Table 3.1). Outer integument becomes hard and forms the outer seed coat, the testa, and the inner integument becomes thin and papery, forming the inner coat, the tegmen. The scar is the place where it is attached with the funicle.
Essay # 10. Apomixis:
During normal sexual cycle, fertilisation (amphimixis i.e., fusion of male and female gametes), leads to the formation of embryo and, later on, seed. The seed germinates to produce new plant. Later on, male and female gametophytes are formed on the plant after meiosis. This normal sexual cycle involves both fertilisation and meiosis. The haploid and diploid phase regularly alternate each other and is known as alternation of generations.
However, in many plants, the normal process of sexual reproduction is substituted by an asexual process. Thus the phenomenon of substitution of sexual process by asexual methods is known as apomixis. The plant which shows the phenomenon is called apomictic plant.
The term ‘apomixis’ was coined by Winkler in 1908. According to him, the term apomixis (i.e., away from mixing) refers to the substitution of sexual reproduction by any such method which does not involve syngamy and meiosis.
It is of the following types:
A. Vegetative reproduction:
In this type new plants are developed from stem, leaf, buds etc. Here seeds are not required.
B. Agamospermy:
In this type, seeds and embryo are formed without meiosis and fertilisation.
It is of the following types:
(i) Adventive embryony:
Embryo is formed from nucellar tissue or integuments.
(ii) Diplospory:
In this type, the megaspore mother cell develops an unreduced embryo sac.
It is of two types:
a. Parthenogenesis:
It is the process of formation of embryo from haploid egg without fertilisation. The resultant plants are haploid, e.g., Solanum nigrum of Solanaceae etc.
b. Apogametry or Apogamy:
It is the process of development of an embryo from any cell except egg of a normal gametophyte (e.g., synergid or antipodal cell), called apogametry or apogamy, e.g., Orchis sp. of Orchidaceae etc.
3. Apospory:
It is the process where the embryo sac develops from a sporophytic cell (2n) of the ovule without any reduction division. So the egg within the diploid embryo sac is diploid, which develops into a diploid embryo. This is also called diploid parthenogenesis. Development of a gametophyte on the sporophyte without reduction division is called apospory, e.g., Eupatorium glandolusum, Taraxacum albidium, several species of Hieracium etc.
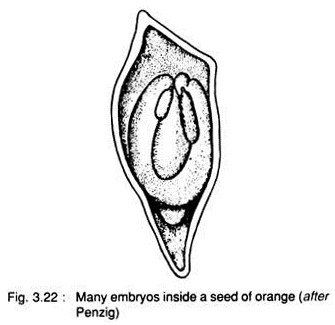
Essay # 11. Polyembryony:
It is the phenomenon of development of more than one embryo inside a seed. Leeuwenhoek (1719) first discovered poly- embryony in orange (Fig. 3.22).
It is of two types:
A. True polyembryony:
When many embryos are developed in the same embryo sac it is called true polyembryony.
B. False polyembryony:
When more than one embryo develop in different embryo sacs inside the ovule it is called false polyembryony.
Polyembryony may develop in seeds by one of the following methods:
1. Cleavage polyembryony:
When more than one embryo is developed by cleavage of zygote it is called cleavage polyembryony. This is commonly found in gymnosperm. In angiosperms, this is found in Nymphea advena, Nicotiana rustica, Erythronium americanum, etc.
2. Embryo from the other embryo sac in the ovule, e.g., Citrus sp. of Rutaceae.
3. Embryo from nucellus i.e., Adventive embryony. Embryos develop from the cells of nucellus which penetrate the embryo sac, e.g., Mangifera indica of Anacardiaceae.
4. Embryo from endosperm, e.g., Balanophora sp. of Balanophoraceae.
5. Embryo from synergids, e.g., Sagittaria sp. of Allismaceae.
6. Embryo from antipodal cell, e.g., Ulmus americana of Ulmaceae.