Wastewater Treatment Essay – This is one of the best essays on ‘Wastewater Treatment’ especially written for school and college students.
Essay on Wastewater Treatment
1. Essay on the Preliminary Treatment of Wastewater:
The purpose of preliminary treatment is to protect the operation of the wastewater treatment plant. This is achieved by removing from the wastewater any constituents which can clog or damage pumps, or interfere with subsequent treatment processes.
Preliminary treatment devices are, therefore, designed to:
1. Remove or to reduce in size the large, entrained, suspended or floating solids. These solids consist of pieces of wood, cloth, paper, plastics, garbage, etc., together with some fecal matter.
2. Remove heavy inorganic solids such as sand and gravel as well as metal or glass. These objects are called grit.
3. Remove excessive amounts of oils or greases.
A number of devices or types of equipment are used to obtain these objectives:
1. Racks and Bar Screens:
These consist of bars usually spaced three-quarter inches to six inches. Those most commonly used provide clear openings of one to two inches. Although large screens are sometimes set vertically, screens are usually set at an angle of 45 to 60 degrees with the vertical. The incoming wastewater is passed through the bars or screens and periodically the accumulated material is removed. The racks or screens may be cleaned either manually or by means of automatically operated rakes. The solids removed by these units can be disposed of by burial or incineration.
2. Comminuting Devices:
Grinders, cutters and shredders. These are devices to break or cut up solids to such size that they can be returned to the wastewater without danger of clogging pumps or piping or affecting subsequent treatment devices. There may be separate devices to grind solids removed by screens or a combination of screen and cutters installed within the wastewater flow channel in such a manner that the objective is accomplished without actually removing these larger solids from the wastewater.
These latter devices are made by a number of manufacturers under various trade names and, in most cases, consist of fixed, rotating or oscillating teeth or blades, acting together to reduce the solids to a size which will pass through fixed or rotating screens or grids having openings of about one-fourth inch. Some of these devices are even designed to operate as a low-lift pump.
Unfortunately, many plants with comminuting devices develop problems within subsequent treatment units due to a buildup of the shredded solids. This is usually witnessed in the aeration system of activated sludge plants. These shredded solids tend to clog diffusers and cling to the impeller blades of mechanical aerators.
3. Grit Chambers:
Wastewater usually contains a relatively large amount of inorganic solids such as sand, cinders and gravel which are collectively called grit. The amount present in a particular wastewater depends primarily on whether the collecting sewer system is of the sanitary or combined type.
Grit will damage pumps by abrasion and cause serious operation difficulties in sedimentation tanks and sludge digesters by accumulation around and plugging of outlets and pump suctions. Consequently, it is common practice to remove this material by grit chambers.
Grit chambers are usually located ahead of pumps or comminuting devices, and if mechanically cleaned, should be preceded by coarse bar rack screens. Grit chambers are generally designed as long channels. In these channels the velocity is reduced sufficiently to deposit heavy inorganic solids but to retain organic material in suspension.
Channel type chambers should be designed to provide controlled velocities as close as possible to 1.0 foot per second. Velocities substantially greater than 1.0 foot per second cause excessive organic materials to settle out with the grit.
The detention period is usually between 20 seconds to 1.0 minute. This is attained by providing several chambers to accommodate variation in flow or by proportional weirs at the end of the chamber or other flow control devices which permit regulation of flow velocity. There are also patented devices to remove grit. One development is the injection of air several feet above the floor of a tank type unit.
The rolling action of the air keeps the lighter organic matter in suspension and allows the grit relatively free from organic matter to be deposited in the quiescent zone beneath the zone of air diffusion. Excessive quantities of air can cause the roll velocity to be too high resulting in poor grit removal. Insufficient quantities of air result in low roll velocities and excessive organic matter will settle with the grit. These grit chambers are usually called aerated grit chambers.
4. Cleaning:
Grit chambers are designed to be cleaned manually or by mechanically operated devices. If cleaned manually, storage space for the deposited grit is usually provided. Grit chambers for plants treating wastes from combined sewers should have at least two hand cleaned units or a mechanically cleaned unit with by-pass.
Mechanically cleaned grit chambers are recommended. Single, hand-cleaned chambers with by-pass, are acceptable for small wastewater treatment plants serving sanitary sewer systems. Chambers other than channel type are acceptable, if provided with adequate and flexible controls for agitation and/or air supply devices and with grit removal equipment.
There are a number of mechanical cleaning units available which remove grit be scrapers or buckets while the grit chamber is in normal operation. These require much less grit storage space than manually operated units.
5. Washing Grit:
Grit always contains some organic matter which decomposes and creates odors. To facilitate economical disposal of grit without causing nuisance, the organic matter is sometimes washed from the grit and returned to the wastewater. Special equipment is available to wash grit. Mechanical cleaning equipment generally provides for washing grit with wastewater as it is removed from the chamber.
6. Quantity of Grit:
This depends on the type of sewer system, the condition of the sewer lines and other factors. Strictly domestic wastewater collected in well-constructed sewers will contain little grit, while combined wastewater will carry large volumes of grit, reaching a peak at times of severe storms. In general, 1.0 to 4.0 cu.ft. of grit per million gallons of wastewater flow can be expected.
7. Operation:
Manually cleaned grit chambers for combined wastewater should be cleaned after every large storm. Under ordinary conditions these grit chambers should be cleaned when the deposited grit has filled 50 to 60 percent of the grit storage space. This should be checked at least every ten days during dry weather.
When mechanically cleaned grit chambers are used, they must be cleaned at regular intervals to prevent undue load on the cleaning mechanism. Recommendations of the manufacturer should be rigidly observed. This plus experience, will determine the cleaning schedule. A grit in which marked odors develop indicates that excessive organic matter is being removed in the grit chamber.
Alternately, if sludge from a settling tank is excessively high in grit, or if there is excessive wear in pumps, comminutors, sludge collectors or other mechanical equipment, the reason is likely to be inefficient functioning of the grit removing process. In either case, a study of this unit should be made.
8. Disposal of Screenings and Grit:
Screenings decompose rapidly with foul odors. They should be kept covered in cans at the screens and removed at least daily for disposal by burial or incineration. The walls and platforms of the screen chamber and screen itself should be hosed down and kept clean. Grit containing much organic matter may have to be buried to prevent odor nuisances.
Pre-Aeration Tanks:
Pre-aeration of wastewater, that is aeration before primary treatment is sometimes provided for the following purposes:
1. To obtain a greater removal of suspended solids in sedimentation tanks.
2. To assist in the removal of grease and oil carried in the wastewater.
3. To freshen up septic wastewater prior to further treatment.
4. BOD reduction.
Pre-aeration is accomplished by introducing air into the wastewater for a period of 20 to 30 minutes at the design flow. This may be accomplished by forcing compressed air into the wastewater at a rate of about 0.10 cu.ft. per gallon of wastewater when 30 minutes of aeration is provided or by mechanical agitation whereby the wastewater is stirred or agitated so that new surfaces are continually brought into contact with the atmosphere for absorption of air. To insure proper agitation when compressed air is forced into the wastewater, air is usually supplied at the rate of 1.0 to 4.0 cubic feet per minute per linear foot of tank or channel.
When air for mechanical agitation (either with or without the use of chemicals) is used for the additional purpose of obtaining increased reduction in BOD, the detention period should be at least 45 minutes at design flow. The agitation of wastewater in the presence of air tends to collect or flocculate lighter suspended solids into heavier masses which settle more readily in the sedimentation tanks. Pre-aeration also helps to separate grease and oil from the wastewater and wastewater solids and to carry them to the surface. By the addition of air, aerobic conditions are also restored in septic wastewater to improve subsequent treatment.
The devices and equipment for introducing the air into the wastewater are the same or similar to those used in the activated sludge process.
Pre-Chlorination:
Pre-chlorination is the chlorination of a wastewater prior to primary treatment. In general, the objectives of pre-chlorination are not related to disinfection, and its use is related to either temporarily preventing further wastewater decomposition or reducing problems associated with wastewater decomposition.
The objectives of pre-chlorination are:
1. Odor control
2. Protection of plant structures
3. Aid in sedimentation, and
4. Reduction or delay of Biochemical Oxygen Demand (BOD).
2. Essay on the Primary Treatment of Wastewater:
Primary treatment is designed to remove organic and inorganic solids by the physical processes of sedimentation and flotation. Primary treatment devices reduce the velocity and disperse the flow of wastewater. In primary treatment the velocity of flow is reduced to 1 to 2 feet per minute to maintain a quiescent condition so that the material denser than water will settle out and material less dense than water will float to the surface.
Approximately 40 to 60 percent of the suspended solids are removed from the waste stream (25-35% BOD reduction). The solids that remain in suspension as well as dissolved solids will usually be biochemically treated in subsequent processes for physical separation and removal in the final (secondary) settling tanks.
The size and number of primary tanks is dependent on the estimated wastewater flow and the design detention time. Generally, a detention time of 2 to 3 hours will provide a sufficient time period for most particles to settle out.
Further, the settling rate of a particle depends on the strength and freshness of the wastewater being treated, the weight of the solid compared to the specific gravity of water, the size and shape of the solid and the temperature of the water. Water is more dense at lower temperatures; therefore, the required settling time increases.
As the temperatures of the water increases, the required settling time decreases. Equal distribution of flow throughout the tank is critical. The greater the velocity in one area, the less the actual detention time. Solids not having sufficient time to settle out will be discharged in the effluent.
Principle primary treatment devices are referred to as sedimentation tanks, primary tanks, primary clarifiers or primary settling tanks, some of which have the further function of providing an additional compartment for the decomposition of settled organic solids which is known as sludge digestion. There are several types of primary tanks in use.
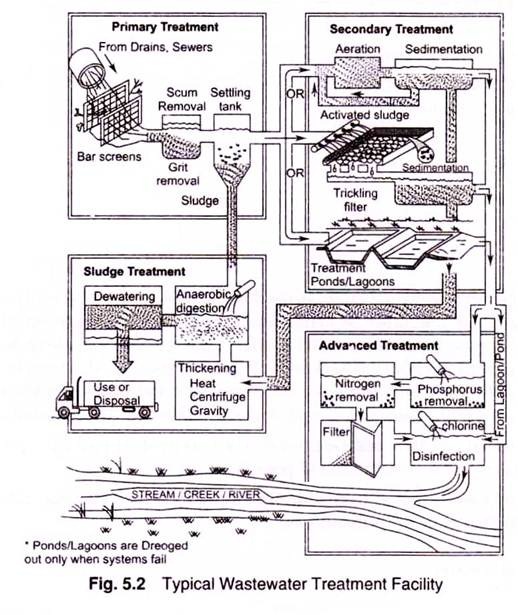
3. Essay on the Secondary Treatment of Wastewater:
Secondary treatment depends primarily upon aerobic organisms which biochemically decompose the organic solids to inorganic or stable organic solids. It is comparable to the zone of recovery in the self-purification of a stream.
The devices used in secondary treatment may be divided into four groups:
1. Trickling filters with secondary settling tanks
2. Activated sludge and modifications with final settling tanks
3. Intermittent sand filters
4. Stabilization ponds.
Sludge Treatment:
The solids removed from wastewater in both primary and secondary treatment units, together with the water removed with them, constitute wastewater sludge. It is generally necessary to subject sludge to some treatment to prepare or condition it for ultimate disposal. Such treatment has two objectives — the removal of part or all of the water in the sludge to reduce its volume, and the decomposition of the putrescible organic solids to mineral solids or to relatively stable organic solids.
This is accomplished by a combination of two or more of the following methods:
1. Thickening
2. Digestion with or without heat
3. Drying on sand bed — open or covered
4. Conditioning with chemicals
5. Elutriation
6. Vacuum filtration
7. Heat drying
8. Incineration
9. Wet oxidation
10. Centrifuging.
Package Units:
The term “package units” is used in the field to describe equipment which has been put on the market by a number of manufacturers that is intended to provide wastewater treatment by the use of prefabricated or modular units. Package units can also refer to a complete installation, including both mechanisms and prefabricated containers. This term is also applied to installations where only the mechanisms are purchased and the containers constructed by the purchaser in accordance with plans and specifications prepared by the manufacturer.
Though specific limitations have not been established, individual package units have, in general, been small installations serving a limited population.
Package units have been adapted to practically all the treatment devices, either singly or in various combinations that have been mentioned.
Disinfection of Wastewater:
Disinfection:
Primary, secondary and even tertiary treatment cannot by expected to remove 100 percent of the incoming waste load and as a result, many organisms still remain in the waste stream. To prevent the spread of waterborne diseases and also to minimize public health problems, regulatory agencies may require the destruction of pathogenic organisms in wastewaters.
While most of these microorganisms are not pathogens, pathogens must be assumed to be potentially present. Thus, whenever wastewater effluents are discharged to receiving waters which may be used for water supply, swimming or shell fishing, the reduction of bacterial numbers to minimize health hazards is a very desirable goal.
Disinfection is treatment of the effluent for the destruction of all pathogens. Another term that is sometimes also used in describing the destruction of microorganisms is sterilization. Sterilization is the destruction of all microorganisms. While disinfection indicates the destruction of all disease causing microorganisms, no attempt is made in wastewater treatment to obtain sterilization. However, disinfection procedures applied to wastewaters will result in a substantial reduction of all microbes so that bacterial numbers are reduced to a safe level.
In general, disinfection can be achieved by any method that destroys pathogens, A variety of physical or chemical methods are capable of destroying microorganisms under certain conditions. Physical methods might include, for example, heating to boiling or incineration or irradiation with X-rays or ultraviolet rays.
Chemical methods might theoretically include the use of strong acids, alcohols, or a variety of oxidizing chemicals or surface active agents (such as special detergents). However, the treatment of wastewaters for the destruction of pathogens demands the use of practical measures that can be used economically and efficiently at all times on large quantities of wastewaters which have been treated to various degrees.
In the past, wastewater treatment practices have principally relied on the use of chlorine for disinfection. The prevalent use of chlorine has come about because chlorine is an excellent disinfecting chemical and, until recently, has been available at a reasonable cost.
However, the rising cost of chlorine coupled with the fact that chlorine even at low concentrations is toxic to fish and other biota as well as the possibility that potentially harmful chlorinated hydrocarbons may be formed has made chlorination less favored as the disinfectant of choice in wastewater treatment. As a result, the increased use of ozone (ozonation) or ultraviolet light as a disinfectant in the future is a distinct possibility in wastewater disinfection.
Both ozone and ultraviolet light, as well as being an effective disinfecting agent, leave no toxic residual. Ozone will additionally raise the dissolved oxygen level of the water. However, ozone must be generated and has only recently begun to compete favorably with chlorination in terms of economics. Ultraviolet light has recently undergone studies to determine its effectiveness and cost when used at large wastewater treatment plants. While the study is not yet complete, ultraviolet light now appears effective and economically competitive with chlorination as a disinfectant.
The use of both chlorine and ozone as chemical disinfectants and their disinfecting properties and actions will be considered individually. However, since chlorine continues to be used extensively as a disinfectant, we will mainly be concerned with the principles and practice of chlorination.
4. Essay on the Tertiary and Advanced Wastewater Treatment:
The terms “primary” and “secondary” treatment have been used to generally describe a degree of treatment; for example, settling and biological wastewater treatment. Since the early 1970s “tertiary” treatment has come into use to describe additional treatment following secondary treatment. Quite often this merely indicates the use of intermittent sand filters for increased removal of suspended solids from the wastewater. In other cases, tertiary treatment has been used to describe processes which remove plant nutrients, primarily nitrogen and phosphorous, from wastewater.
Improvement and upgrading of wastewater treatment units as well as the need to minimize environmental effects has led to the increased use of tertiary treatment.
A term that is also sometimes used to indicate treatment of a wastewater by methods other than primary or biological (secondary) treatment is advanced treatment. This degree of treatment is usually achieved by chemical (for example coagulation) methods as well as physical methods (flocculation, settling and activated carbon adsorption) to produce a high quality effluent water.
Types of Advanced Wastewater Treatment:
Advanced Wastewater Treatment may be broken into three major categories by the type of process flow scheme utilized:
1. Tertiary Treatment
2. Physical-Chemical Treatment
3. Combined Biological-Physical Treatment.
Tertiary treatment may be defined as any treatment process in which unit operations are added to the flow scheme following conventional secondary treatment. Additions to conventional secondary treatment could be as simple as the addition of a filter for suspended solids removal or as complex as the addition of many unit processes for organic, suspended solids, nitrogen and phosphorous removal.
Physical-chemical treatment is defined as a treatment process in which biological and physical-chemical processes are intermixed to achieve the desired effluent. Combined biological-physical-chemical treatment is differentiated from tertiary treatment in that in tertiary treatment any unit processes are added after conventional biological treatment, while in combined treatment, biological and physical-chemical treatment are mixed.
Another way to classify advanced wastewater treatment is to differentiate on the basis of desired treatment goals.
Advanced wastewater treatment is used for:
(i) Additional organic and suspended solids removal.
(ii) Removal of Nitrogenous Oxygen Demand (NOD).
(iii) Nutrient removal.
(iv) Removal of toxic materials.
(v) Advanced wastewater treatment plant effluents may be recycled directly or indirectly to increase the available domestic water supply.
(vi) Advanced wastewater treatment effluents may be used for industrial process or cooling water supplies.
(vii) Some receiving waters are not capable of withstanding the pollutional loads from the discharge of secondary effluents.
(viii) Secondary treatment does not remove as much of the organic pollution in wastewater as may be assumed.
The first three reasons for additional organic removal through advanced wastewater treatment are simple. The fourth requires some explanation. The performance of secondary treatment plants is almost always measured in terms of BOD and SS removals. A well designed and operated secondary plant will remove from 85 to 95% of the influent BOD and SS. However, the BOD test does not measure all of the organic material present in the wastewater.
An average secondary effluent may have a BOD of 20 mg/L and a COD of 60 to 100 mg/L. The average secondary plant removes approximately 65% of the influent COD. Thus, when high quality effluents are required, additional organic removals must be accomplished. In addition to the organic materials remaining in most secondary effluents, there is an additional oxygen demand resulting from the nitrogen present in the wastewater.
In wastewaters, much of the nitrogen is found in the form of ammonia. When secondary treatment is used, a great deal of this ammonia is discharged in the effluent. Bacteria can utilize this ammonia as an energy source and convert ammonia to nitrite and nitrate.
NH3+ O2 + Bacteria NO2 + O2 + Bacteria NO3
Another reason for advanced wastewater treatment may be to remove nutrients contained in discharges from secondary treatment plants. The effluents from secondary treatment plants contain both nitrogen (N) and phosphorous (P). N and P are ingredients in all fertilizers. When excess amounts of N and P are discharged, plant growth in the receiving waters may be accelerated.
Algae growth may be stimulated causing blooms which are toxic to fish life as well as aesthetically unpleasing. Fixed plant growth may also be accelerated causing the eventual process of a lake becoming a swamp to be speeded up. Therefore, it has become necessary to remove nitrogen and phosphorous prior to discharge in some cases.
Toxic materials, both organic and inorganic are discharged into many sewage collections systems. When these materials are present in sufficient quantities to be toxic to bacteria, it will be necessary to remove them prior to biological treatment. In other cases, it is necessary to remove even small amounts of these materials prior to discharge to protect receiving waters or drinking water supplies.
Thus, advanced wastewater treatment processes have been used in cases where conventional secondary treatment was not possible due to materials toxic to bacteria entering the plant as well as in cases where even trace amounts of toxic materials were unacceptable in plant effluents.
Nitrification:
Biological nitrification may be used to prevent oxygen depletion from nitrogenous demand (NOD) in the receiving waters. Nitrification is simply the conversion of ammonia to nitrate in the treatment plant rather than in the receiving water. Nitrification may be carried out in the same tank as BOD removal or in a separate stage. Nitrification may be carried out either in activated sludge flocs or in fixed films.
Regardless of the particular scheme chosen, the same basic requirements for nitrification must be maintained:
(i) Oxygen level
(ii) Loading rates
(iii) Solids retention time
(iv) Alkalinity
(v) pH
(vi) Freedom from toxic materials
(vii) Temperature.
Sufficient oxygen must be available for nitrification to occur. Approximately 4.5 pounds of dissolved oxygen are required for the conversion of 1 pound of ammonia to nitrate. Dissolved oxygen sufficient to satisfy the remaining BOD is also required. In activated sludge plants the mixing requirement of the basins must also be considered. Generally, dissolved oxygen levels of approximately 2-3 mg/L are recommended for nitrification. The bacteria responsible for nitrification reproduce at a much slower rate than those responsible for BOD removal.
Thus, the danger always exists for the “wash out” of the nitrifying organisms. That is, unless the nitrifying bacteria reproduce at the same or greater rate than they are removed from the system (by waste sludge) then the population of bacteria will be insufficient to carry out nitrification. For this reason, nitrification systems are operated at higher return sludge rates than conventional secondary treatment. The amount of sludge to be wasted is significantly less than from a conventional activated sludge system.
Nitrification systems are sensitive to pH variation. Optimum pH has been found to be approximately 7.8 to 9.0. Reductions in nitrification have been found outside this range. Alkalinity is also destroyed during nitrification. Theoretically, 7.2 pounds of alkalinity are destroyed in converting 1 pound of ammonia to nitrate. In low alkalinity wastewaters, Quick lime (CaO) or Ca(OH)2 is often used to provide alkalinity and pH control.
Generally, the influent BOD to nitrification systems has not been found to effect performance. However, sufficient oxygen must be provided for the organic demand and organic shock loads must be avoided.
Biological Denitrification:
Biological nitrification satisfies the nitrogenous oxygen demand by converting NH3 to NO3. In some applications, such as discharge into enclosed bodies of water or recycle to water supplies, nitrification may not be sufficient. When nitrogen removal is required, one of the available methods is to follow biological nitrification with biological denitrification.
Denitrification is accomplished under anaerobic or near anaerobic conditions by bacteria commonly found in wastewater.
Nitrates are removed by two mechanisms:
1. Conversion of NO3 to N2 gas by bacterial metabolism and
2. Conversion of NO3 to nitrogen contained in cell mass which may be removed by settling.
In order for denitrification to occur, a carbon source must be available. Most commonly, methanol is used. The methanol must be added in sufficient quantity to provide for cell growth and to consume any dissolved oxygen which may be carried into the denitrification reactor.
Usually 3 to 4 pounds of methanol per pound of nitrate are required. Careful control of methanol feed is necessary to prevent waste of chemicals. In addition, if excess methanol is fed to the system, unused methanol will be carried out in the effluent causing excessive BOD.
Denitrification may be carried out in either a mixed slurry reactor or in fixed bed reactors. Denitrification filters carry out both denitrification and filtration in the same unit. Mixed slurry systems consist of a denitrification reactor, re-aeration basin and clarifiers. Re-aeration prior to clarification is required to free the sludge from trapped bubbles of nitrogen gas.
Denitrifying bacteria grow very slowly and are extremely sensitive to temperature.
Denitrification rates have been shown to increase five-fold when the temperature is increased from 10 °C to 20 °C. Thus, operating parameters such as sludge age and retention time must be varied with temperature.
The pH in denitrification systems must be carefully controlled. The optimum pH is from 6.0 to 8.0.
Denitrification is a very sensitive and difficult process to operate. Little full scale operational experience is available. Constant monitoring of pH, methanol feed and temperature is essential to successful operation.
Filtration:
Granular media filtration to remove those suspended and colloidal solids which are carried over from previous unit processes is a common unit process in advanced wastewater treatment.
Effluents of less than 10 mg/L BOD and 5 mg/L suspended solids are not uncommon for effluents from biological treatment processes after filtration.
Gravity filters similar to rapid sand filters are sometimes used. Often a combination of filter medias, such as anthracite coal and sand are used to provide coarse to fine filtration as the water passes through the filter. The water passes through the filter media and support gravel and is then collected by the under drain system. As filtration proceeds, the head loss through the filter increases until it reaches an unacceptable level or until solids breakthrough occurs and the effluent becomes unacceptable. When either the head loss becomes excessive or solids breakthrough occurs, the filter is backwashed.
Gravity filters are generally run at 1.5 to 2.5 gpm per square foot. Pressure filters are used to obtain filter rates up to 6 gpm per square foot. Ideally, filters are designed to have the solids in the effluent and the head loss reach their allowable levels at the same time.