Read this essay to learn about Soil Pollution. After reading this essay you will learn about: 1. Definition of Soil Pollution 2. Causes of Soil Pollution 3. Kinds 4. Effects 5. Control.
Contents:
- Essay on the Definition of Soil Pollution
- Essay on the Causes of Soil Pollution
- Essay on the Kinds of Soil Pollution
- Essay on the Effects of Soil Pollution
- Essay on the Control of Soil Pollution
Essay # 1. Definition of Soil Pollution:
Soil is the thin layer of organic and inorganic materials that covers the Earth’s rocky surface. The organic portion, which is derived from the decayed remains of plants and animals, is concentrated in the dark uppermost topsoil.
The inorganic portion made up of rock fragments, was formed over thousands of years by physical and chemical weathering of bedrock. Productive soils are necessary for agriculture to supply the world with sufficient food.
Soil pollution is defined as persistent of toxic compounds, chemicals, salts, radioactive materials, or disease causing agents, which have adverse effects on plant growth and animal health.
Soil becomes polluted by:
i. Seepage from a landfill.
ii. Discharge of industrial waste into the soil.
iii. Percolation of contaminated water into the soil.
iv. Rupture of underground storage tanks.
v. Excess application of pesticides, herbicides or fertilizer.
vi. Solid waste seepage.
vii. Chemicals like petroleum hydrocarbons, heavy metals, pesticides and solvents.
Essay # 2. Causes of Soil Pollution:
A soil pollutant is any factor which deteriorates the quality, texture and mineral content of the soil or which disturbs the biological balance of the organisms in the soil. Pollution in soil has adverse effect on plant growth.
Mainly soil pollution is caused by the presence of man-made chemicals application of pesticides, percolation of contaminated surface water to subsurface strata, oil and fuel dumping, leaching of wastes from landfills or direct discharge of industrial wastes to the soil.
The most common chemicals involved are petroleum hydrocarbons, solvents, pesticides, lead and other heavy metals. Occurrence of this phenomenon is correlated with the degree of industrialization and intensities of chemical usage.
The main causes of soil pollution are described below in details:
i. Haphazard Use of Fertilizer:
Soil nutrients are vital for plant growth and development. Plants obtain carbon, hydrogen and oxygen from air and water. All other necessary nutrients like nitrogen, phosphorus, potassium, calcium, magnesium, sulfur and more must be obtained from the soil.
Farmers generally use fertilizers to correct soil deficiencies. Fertilizers contaminate the soil with impurities, which come from the raw materials used for their manufacture. Mixed fertilizers often contain ammonium nitrate (NH4NO3), phosphorus as P2O5, and potassium as K2O.
For instance, As, Pb and Cd present in traces in rock phosphate mineral get transferred to super phosphate fertilizer. Since the metals are not degradable, their accumulation in the soil above their toxic levels due to excessive use of phosphate fertilizers becomes an indestructible poison for crops.
Fertilizers are very valuable, as they replace the soil nutrients used up by plants. The three primary soil nutrients often in short supply are potassium, phosphorous and nitrogen (NPK) compounds. These are commonly referred to as macronutrients. Certain other elements like boron, zinc and manganese are necessary in extremely small amounts and are known as micronutrients.
When crops are harvested, a large amount of macronutrients and a small amount of micronutrients are removed with the crops. If the same crop is grown again, depleted levels of the nutrients can result in decreased yields. These necessary nutrients can be returned to the soil through the application of fertilizers.
The over use of NPK fertilizers reduce quantity of vegetables and crops grown on soil over the years. It also reduces the protein content of wheat, maize, grams, etc., grown on that soil.
The carbohydrate quality of such crops also gets degraded. Excess potassium content in soil decreases vitamin C and carotene content in vegetables and fruits. The vegetables and fruits grown on over- fertilized soil are more prone to attacks by insects and disease.
Approximately 25% of the world’s crop yield is estimated to be directly attributed to the use of chemical fertilizers. The use of chemical fertilizers has increased significantly over the last few decades and is expected to rise even higher.
ii. Indiscriminate use of Pesticides, Insecticides and Herbicides:
In addition to fertilizers, a large amount of pesticides (chemicals used to kill or control populations of unwanted fungi, animals or plants often called pests) are also used to ensure a good yield. Pesticides can be subdivided into several categories, based on the kinds of organisms they are used to control.
Insecticides are used to control insect populations, while fungicides are used to control unwanted fungal growth. Mice and rats are killed by rodenticides, while plant pests are controlled by herbicides. The first widespread insecticide use began at the end of World War-IT and included DDT (dichlorodiphenyltrichloroethane) and gammaxene.
Insects soon became resistant to DDT and as the chemical did not decompose readily, it persisted in the environment. Since it was soluble in fat rather than water, it biomagnified up the food chain and disrupted calcium metabolism in birds, causing eggshells to be thin and fragile.
As a result, large birds of prey such as the brown pelican, ospreys, falcons and eagles became endangered. DDT has been now banned in most western countries. Ironically many of them including USA still produce DDT for export to other developing nations whose needs outweigh the problems caused by it.
The most important pesticides are DDT, BHC, chlorinated hydrocarbons, organophosphates, aldrin, malathion, dieldrin, furodan, etc. The remnants of such pesticides may get adsorbed by the soil particles, which then contaminate root crops grown in that soil. The consumption of such crops causes the pesticides remnants to enter human biological systems, affecting them adversely.
An infamous herbicide used as a defoliant in the Vietnam War called Agent Orange (dioxin), was eventually banned. It had caused cancer, skin conditions and infertility in soldiers.
Pesticides not only bring toxic effect on human and animals but also decrease the fertility of the soil. Some of the pesticides are quite stable and their bio- degradation may take weeks and even months.
For example, DDT, one of the first synthetic organic insecticides to be used, was thought to be the perfect insecticide. During the first ten years of its use (1942-1952), DDT is estimated to have saved about five million lives primarily because of its use to control disease-carrying mosquitoes.
However, after a period of use, many mosquitoes and insects became tolerant to DDT, thus making it lose its effectiveness. In temperate regions, DDT has a half-life (the amount of time required for half of the chemical to decompose) of 10-15 years. This means that if 100 kg of DDT were to be sprayed over an area, 50 kg would still be present in the area 10-15 years later.
The half-life of DDT varies according to the soil type, temperature, kind of soil organisms present, and other factors. In tropical parts of the world, the half-life may be as short as 6 months. Persistent pesticides become attached to small soil particles which are easily moved by wind and water to different parts thus affecting soils elsewhere.
Persistent pesticides may also accumulate in the bodies of animals, and over a period of time increase in concentration if the animal is unable to flush them out of its system, thus leading to the phenomenon called bioaccumulation. When an affected animals is eaten by another carnivore, these pesticides are further concentrated in the body of the carnivore.
This phenomenon of acquiring increasing levels of a substance in the bodies of higher trophic level organisms is known as ‘bio-magnification’. This process, especially in the case of insecticides like DDT, has been proved to be disastrous.
iii. Dumping of Solid Wastes:
In general, solid waste includes garbage, domestic refuse and discarded solid materials such as those from commercial, industrial and agricultural operations. They contain a large amounts of paper, cardboards, plastics, glass, old construction material, packaging material and toxic or otherwise hazardous substances.
a. Waste dumps:
Land gets dumping of industrial wastes, municipal wastes, medicals or hospital wastes. Industrial solid wastes and sludge are the major sources of soil pollution by toxic organic and inorganic chemical compounds and heavy metals.
The fall-out from industrial emissions, for example the fly ash emitted by thermal power plants, can pollute surrounding lands. We must keep in mind that the particulates of the industrial emissions from the tall chimney always come back to the earth surface sooner or later.
Radioactive tests from nuclear testing laboratories and nuclear power plants and the radioactive fall-out from the nuclear explosions also contaminate the soil. Radioactive materials thrive in the soil for long periods because they usually have a long half-life. Stroncium-90, for example, has a half-life of 28 years, and the half-life of Caesium-137 is 30 years.
b. Municipal wastes:
Municipal wastes mainly include domestic and kitchen wastes, market wastes, hospital wastes, livestock and poultry wastes, slaughterhouse wastes, waste metals, glass and ceramic wastes, etc. Non-biodegradable materials like used polyethylene, carry bags, waste plastic sheets and bottles etc. persist in soil for long periods.
Hospital wastes contain organic materials, chemicals, metal needles, plastic and glass bottles, vials, etc. Dumping of domestic sewage and hospital organic wastes contaminate the environment with a variety of pathogens that can seriously affect human health.
The portion of solid waste that is hazardous such as oils, battery metals, heavy metals from smelting industries and organic solvents are the ones we have to pay particular attention to.
These can in the long run, get deposited to the soils of the surrounding area and pollute them by altering their chemical and biological properties. More than 90% of hazardous waste is produced by chemical, petroleum and metal-related industries and small businesses such as dry cleaners and gas stations contribute as well.
Toxic chemicals leached into the soil underneath homes, causing an unusually large number of birth defects, cancers and respiratory, nervous and kidney diseases.
iv. Deforestation:
Soil erosion occurs when the weathered soil particles are dislodged and carried away by wind or water. Deforestation, agricultural development, temperature extremes, precipitation including acid rain, and human activities contribute to this erosion. Humans speed up this process by construction, mining, cutting of timber, over cropping and overgrazing. It results in floods and cause soil erosion.
Forests and grasslands are an excellent binding material that keeps the soil intact and healthy. They support many habitats and ecosystems, which provide innumerable feeding pathways or food chains to all species. Their loss would threaten food chains and the survival of many species.
During the past few years quite a lot of vast green land has been converted into deserts. The precious rain forest habitats of South America, tropical Asia and Africa are coming under pressure of population growth and development.
Soil erosion occurs when the worn out particles are dislodged and passed away by wind or water. Deforestation, agricultural development, and human actions add to this erosion.
Forests hold up many habitats and ecosystems, which make available immeasurable feeding pathways or food chains to all species. During the past few years quite a lot of vast green land has been converted into deserts. Deforestation is slowly destroying the most dynamic flora and fauna areas.
Essay # 3. Kinds of Soil Pollution:
There are generally five different kinds of pollution namely:
1. Pesticides pollution in soil— those are mostly used as soil application for agricultural purposes and all of which reach the soil,
2. Inorganic contaminants or pollutants—mostly heavy metal pollution in the soil,
3. Organic wastes—those from concentrated feed lots and food processing plants along with municipal and industrial wastes, some of which may be dumped on soil,
4. Fertilizers and other salts—contamination of soils with different soluble salts is one form of soil pollution primarily agricultural in origin, and
5. Radio-nuclides—nuclear fission associated with atomic weapons testing provides another kind of soil pollution.
1. Pesticides:
Pesticides, legally termed economic poisons, include insecticides, fungicides, herbicides, rodenticides and nematodes and many other chemical like disinfectants, antibiotics, defoliants, chemosterilants and juvenile hormones etc. Many of these chemicals are deadly—not only to the intended or particular organisms but also to other life forms, including people.
Some can be accumulated by lower organisms (aquatic micro-organisms, plant life) and increase in concentration successively up the food chain until toxic concentrations are consumed by the higher animals (birds, mammals).
This increase in concentration up the food chain is called biological magnification. Many toxic pesticides of group organo-phosphates are active in the environment only a few days or a week or so. Soil micro-organisms decompose these groups of pesticides as they do other organic materials.
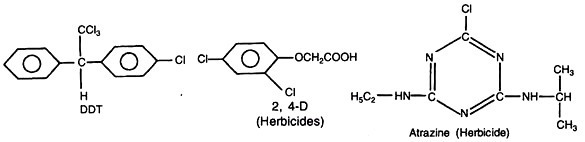
Some commonly used pesticides are shown below:
When these are applied in soils, they undergo following reactions:
(i) Chemicals may vaporize and be lost to the atmosphere without chemical changes,
(ii) May be absorbed by soil colloids,
(iii) May move downward through the soil in liquid or solution form and be lost by leaching,
(iv) May undergo various chemical reactions within the soil environment or on the soil surface, and
(v) May be degraded by soil micro-organisms.
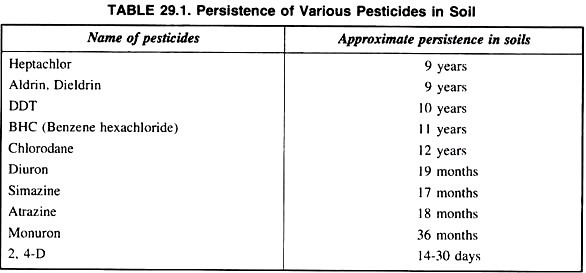
A major concern particularly about chlorinated hydrocarbons like DDT, aldrin, heptachlor, etc. is their persistence in soil and their movement into water streams through soil erosion and entry in the food chain of various wild life. Their movement in soil, leeching water and absorption by plants and changes in microbiological activity in soil are of great concern. The persistence of various pesticides is given in the table 29.1.
Some of these above pesticides are very persistent in soils; hence cause of concern to human beings. The persistence of pesticides in soils is a summation of all reaction, movements and bio-degradation affecting pesticides.
The hazards due to pesticide pollution, however, may be somewhat reduced with the application of organic matter in the soil. Sometimes chemical degradation of DDT, aldrin, heptachlor, malathion, diazinon and atrazine reduced the intensity of toxicity of those pesticides. In addition bio-degradation by soil micro-organisms is the most important method by which pesticides are removed from soils.
2. Inorganic Contaminants:
The trace elements concentrated in a irregular way are usually metals especially the heavy metals (density >6 g cc-1). Some are essential for life processes but all are toxic to organisms at higher concentrations. Seventy six (76) trace elements all of which are potential pollutants.
There are number of inorganic contaminants including heavy metals like, Hg, Cd, Pb, As, Nl, Cu, Zn, Mn etc. Among these Cd and as are extremely poisonous. Mercury, Pb and Ni are moderately poisonous and Cu, Zn and Mn are relatively lower in toxicity.
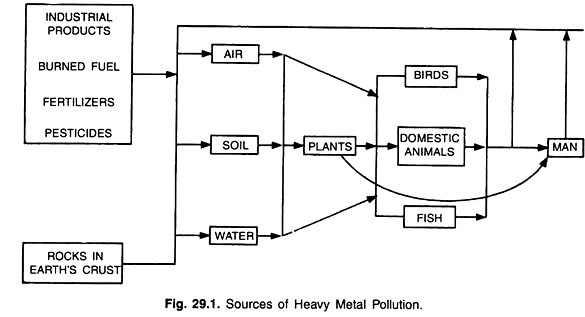
The sources of heavy metal pollution in the soil-water-air-organisms ecosystem are given below:
Different beneficial microbial processes in soil and interaction, both positive and negative may be affected or sometimes altered by the presence of high concentrations of different heavy metals like Cd, Pb, Hg, As, Ni etc. in soil. Besides their phytotoxic effect, high levels of those heavy metals may disturb the nutrient balance in soil and thereby affect the biochemical processes and crop yields.
The phytotoxic effect of heavy metals in soils especially Cd is known to be controlled by various mechanisms. Such as exchange reactions, chelation by organics, absorption by colloidal oxides and hydroxides of iron and manganese etc.
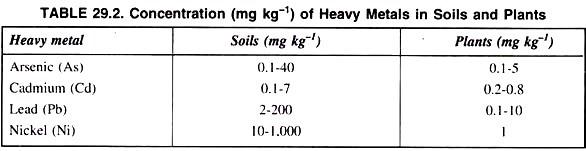
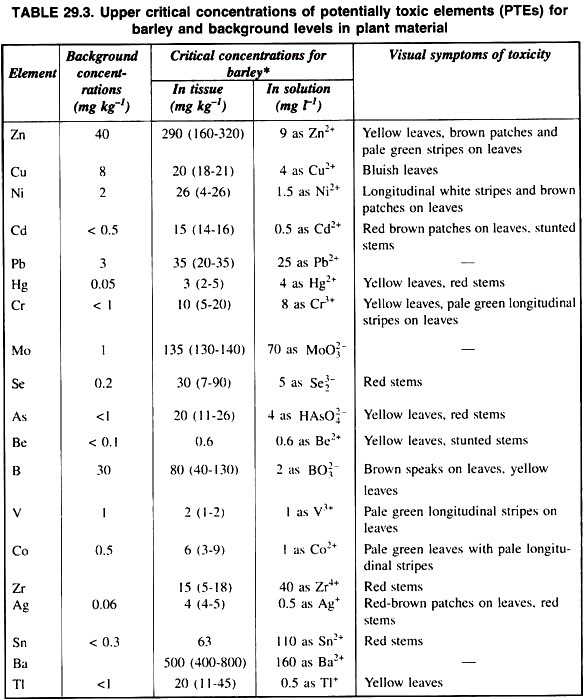
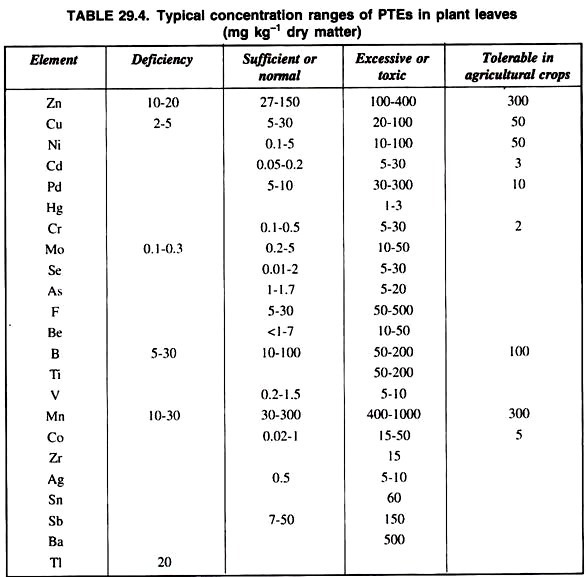
Besides these, soil, contamination due to inorganic pollutants may be alleviated by the elimination of soil application and recycling of toxins in soils. A list of concentration of different heavy metals in soil and plants is presented in table 29.2.
Zinc, Copper, Manganese and Nickel:
The reaction of these elements in soil is certainly affected by the pH, organic matter content, and, the redox status of the soil. The tendency of these cations is to form’ ‘chelate” in the presence of organic matter which influences their behaviour in soils. The relative strength of chelation is generally Copper > Nickel > Zinc > Manganese.
Lead:
Soil lead is largely unavailable to plants. As with the other toxic metallic cations, lead is quite insoluble in soil, especially if the soil is not too acid. The maximum amount of lead was found to be concentrated on the soil surface. Liming reduces the availability of lead and its uptake by plants. Therefore, lead does not create so much of problem in the phytotoxicity.
Arsenic:
Application of arsenical pesticides over a long period results accumulation of arsenic in soils to a toxic level and creates phytotoxicity. Arsenic behaves in soils like that of phosphate. Arsenic present in an anionic form (AsO4-3) and it is adsorbed by oxides and hydrous oxides of Fe and Al.
This so adsorbed arsenate ion is replaceable from these oxides by the phosphate (PO43-) anion through anion exchange mechanism. The toxicity of arsenic can be minimised with the application of sulphate salts to Zn, Fe and Al which can form their insoluble arsenate compounds unavailable to plants.
Cadmium:
Cadmium is extremely phytotoxic as well as poisonous to human beings. The toxicity of cadmium (Cd) is dependent on the dynamic interactions that occur between the pollutant, the environment and the biota. Cadmium tends to accumulate in plant tissues at concentrations exceeding that of the soil solution. Besides its phytotoxic effect, high levels of Cd in soils may disturb the nutrient balance in soil.
Various physico-chemical properties of soils the pH, temperature, organic matter, clay content, moisture content etc. also appear to influence Cd toxicity. Cadmium is most mobile in acid soil whereas in alkaline soil, Cd is immobile.
Cadmium seemed less strongly associated with high molecular weight organics and hydrous oxides of Fe and Al and thus it is readily accumulated by plants grown in Cd-contaminated soils. The phytotoxic effect of Cd can be reduced with the application of organic matter and also with the application of micro-nutrient fertilizers like Zn and Cu which may interact with Cd rendering unavailable to plants.
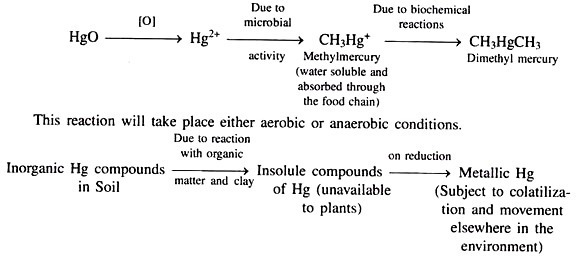
Mercury:
Metallic mercury is first oxidised by the following chemical reaction in the sediment layer of lakes and streams:
Mercury is not readily absorbed by plants from soils unless it is in the methyl mercury form.
Heavy Metal Uptake:
The application of potentially toxic elements (PTEs) to the soil through composts, sewage-sludge and other waste materials may increase heavy metals concentration in crops, but has little or no effect on others depending on the chemistry and behaviour of particular heavy metals in soil and subsequent partitioning within the plant.
It has been found that the application of waste materials including sewage-sludge increased PTEs concentration in crops in the decreasing order: Zn > Cd > Ni > Cu >Pb = Hg = Cr. However, certain PTEs clearly accumulate in crop plants grown in soil more readily than other sludge or other wastes contaminants and in particular Zn, Cu and Ni are readily absorbed to potentially phytotoxic levels.
Recommendation for the regulation of Zn, Cu and Ni in sludge-amended agricultural soils implied that the relative phytotoxic responses of Zn/Cu/Ni may be additive and then the amount of toxic metal in sludge/any other waste contaminants may be expressed as a single term known as the “Zn-equivalent” and it may be obtained by adding together the Zn content, 2 × the Cu content and 8 × the Ni content.
Zn-equivalent = Zn content + 2 × Cu content + 8 × Ni content.
However, this Zn-equivalent model may not be applicable over a wider range of plant species. Zinc, Cu and Ni are the principal phytotoxic elements applied to soil in sludge, but current soil limit values for these elements ensure uptake into crops remains below the critical toxic concentration thresholds in plant tissues.
The maximum permissible soil concentrations were established using sensitive crops and coarse textured soils in field and pot culture studies, and therefore, the limits protect all crops grown on a range of soil types from phytotoxicity.
The soil limits are adjusted according to soil pH values to further minimise the risk of phytotoxicity. Organic contaminants have no phytotoxic activity at the low concentrations. If sewage-sludge is treated in a sludge treatment plant before its use to the soil application, then on its application, crop yields increase through the improvement of soil physical properties.
Sludge-borne organic contaminants represent a relatively minor risk to soil fertility compared with agricultural pesticides. However, the beneficial effects of sludge organic matter and nutrients in improving soil fertility and productivity have been extensively reported and are universally recognised.
The incorporation or injection of sludge protects the natural ecosystems by preventing environmental problems caused by run-off and volatilization.
3. Organic Wastes:
The pollution potential of organic wastes, urban and rural, has become a national and even international problem especially in relation to soil pollution because organic wastes contain detergents, water softeners, borates, phosphates etc.
Sewage effluents, agricultural wastes like animal and crop residues etc. cause a serious soil pollution. Sludge also contributes soil pollution through the supply of live viruses, viable intestinal worm eggs, high concentrations of soluble salts, various heavy metals etc.
Different organic waste materials are rich source for building up soil fertility if it is recycled in agricultural lands after compositing but the main problem is its disposal because of its bulky nature and the huge cost of transport.
The non-biodegradable materials and solid non-decomposable wastes make the problem more complicated.
4. Fertilizers and Other Soluble Salts:
The accumulation of excess salts either through the application of chemical fertilizers or through the application of irrigation water threatens production of crops particularly in arid or semi-arid regions. The concentration of fluorine, selenium and boron in water also increases due to development of soil salinity and alkalinity and pose a problem to human beings and animals.
Fertilizers used to increase the growth of crops also increase algal growth in surface waters into which they are washed. This increased water fertility, which causes accelerated algae and water plant growth, is called eutrophication. Most often increased amounts of phosphorus and nitrate nitrogen are the cause of eutrophication.
Soils heavily fertilized above levels recommended and soil naturally high infertility are potential sources of nitrate contamination in ground or run-off waters.
On the other hand, the major source of phosphorus contamination in surface waters comes from direct dumping of wastes (sewage, animal wastes industrial wastes) and from-eroded suspended soils from urban and agricultural lands. Eroding soil and other phosphorus carrying solids continually supply phosphorus to waters.
Accumulation of sufficient amount of salts in the plant root zone may be a serious problem due to application of salt-laden irrigation water in soil. In heavily populated and industrialised areas where water is returned to streams following its domestic or individuals use. Salts in the sewage and salts leached from watershed soils combinedly increase the level of salts to a great extent.
Thus, fertilizer use including organic nutrient sources does increase nutrient contamination in run-off waters and ground waters. Unnecessary pollution should be avoided by wise use of fertilizers, but zero contamination is impossible, unrealistic and catastrophic to the production of food supplies.
Most dissolved inorganic chemicals in natural waters are soluble salts. They are found in all solutions. In high concentrations salts are unwanted because they reduce or inhibit plant growth, make drinking water unpalatable and interfere in many other uses of water.
Salt pollution from agricultural run-off water is mostly non-point pollution (which means the pollution does not always derive from a single source or point but from various sources).
5. Radio Nuclides:
The main concern with the radio-activity is the fall out of radio-active materials produced from explosions and hazards associated with it the other source of pollution is through the dumping of the waste materials from the nuclear power plants, nuclear reactors and wastes from the medical and other research laboratories.
The most important and long lived ratio-active elements in soils are strontium-90 (half-life = 28 years) and cesium-137 (half-life = 30 years). In normal soils it is not to be hazardous, but in the event of a catastrophic supply of fission products could toxic soil levels of 90Sr and 37Cs be expected.
These radio-active elements could be decontaminated by continuous cropping, removal of surface soil, deep ploughing, leaching, use of fertilizers and amendments. The use of chelates like EDTA, FEDTA, and DTPA in conjunction with algal and fungal cells is most effective in reducing radioactivity from the soil.
Essay # 4. Effects of Soil Pollution:
Land pollution effects will be and still are at the edge of being fatal! Below mentioned are the major effects of soil pollution and how it affects all living things.
a. Effects on Agriculture:
Everything that we consume in order to live is originated from agriculture. Soil being the beginning of a healthy agriculture.
The major effects of soil pollution on agriculture are:
i. Loss of nutrients in the soil
ii. Soil erosion
iii. Less fertile land for vegetation
iv. Reduction in crop yield
v. Reduction in nitrogen fixation.
b. Effects on Ecosystem:
Contamination of soil will definitely have adverse effects on the ecosystem which are as follows:
i. Ecological imbalance
ii. Permanent change in the chemical properties of soil
iii. Alteration in the metabolism of endemic microorganisms resulting in eradication of the primary food chain which is interred related with the existence of each and every organism in the world, including humans
iv. Bad health in all consumers. It is already evident that due to consumption of DDT effected crops, the egg shells of hens have become weak.
c. Effects on Human:
Below mentioned are some such effects of soil pollution, which are visible at some places.
i. Pollution in drinking water
ii. Contamination in vegetation due to presence of chemicals
iii. Problems of waste management
iv. Polluted environment with harmful gases to breathe in and foul smells
v. Health issues
d. Long Term Effects of Soil Pollution:
Soil that has been contaminated should no longer be used to grow food, because the chemicals can leech into the food and harm people who eat it.
If contaminated soil is used to grow food, the land will usually produce lower yields than it would if it were not contaminated. This, in turn, can cause even more harm because a lack of plants on the soil will cause more erosion, spreading the contaminants onto land that might not have been tainted before.
In addition, the pollutants will change the makeup of the soil and the types of microorganisms that will live in it. If certain organisms die off in the area, the larger predator animals will also have to move away or die because they’ve lost their food supply. Thus it’s possible for soil pollution to change whole ecosystems.
Essay # 5. Control of Soil Pollution:
There are different types of soil pollution, namely agricultural soil pollution, industrial waste causing soil pollution, urbanization causing soil pollution. These different types of pollution cause the fertility of the soil to reduce and mineral content in the soil to be destroyed. Therefore, measures have to be taken for preventing soil pollution.
i. Use Bio- Fertilizers:
To increase agricultural yield, most farmers use chemical fertilizers. No doubt that the yield did indeed increase, but at the cost of the soil losing its fertility. To restore the fertility of the soil, the farmers should be encouraged to start using bio-fertilizers. The microorganisms in these fertilizers will help in increasing the fertility of the soil.
ii. Use Bio- Pesticides and Fungicides:
To avoid soil pollution, it is important, that along with fertilizers, farmers should also use to bio pesticides and fungicides. These products will take a little longer to react, but they do not have adverse effect on the soil.
iii. Reduce Toxic Waste:
If one has to look at the soil pollution facts, it will be seen that toxic waste has a big role to play in soil pollution. Hence, industrial toxic waste should be treated to reduce its toxicity before it is disposed of. At the same time, responsible methods should be used for disposing off the waste. The best however, is to avoid the use of harmful chemicals unless they are of extreme importance.
iv. Recycle Waste:
Although a lot of propaganda has been carried out about recycling waste, not many measures have been taken about the same. If each family has to take it upon themselves to recycle waste, the land pollution cause due to land-fills will be reduced considerably. The land so saved can be used constructively for a number of better tasks.
v. Reuse:
After plastic was invented, people thought it was convenient to opt for plastic containers, bags, etc., which could be disposed of after use. However, plastic is one of the main causes of soil pollution, as it takes a very long time to disintegrate. Therefore, people should consider shifting to reusable containers like glass, cotton bags, etc.
Although paper does disintegrate faster, a lot of trees are cut for producing paper bags. Therefore, it is best to opt for cloth bags. Similarly, instead of using tissue papers in the kitchen, etc., one should opt using cloth napkins, handkerchief, etc. This will go a long way in reducing land-fills.
vi. Deforestation:
To prevent soil pollution, deforestation measures have to be undertaken at rapid pace. Soil erosion is caused, when there are no trees to prevent the top layer of the soil from being transported by different agents of nature like water and air. At the same time, measures should be taken to avoid over cropping and over grazing, as it leads to flood and soil erosion and further deterioration of the soil layer.
vii. Reforesting:
Control of land loss and soil erosion can be attempted through restoring forest and grass cover to check wastelands, soil erosion and floods. Crop rotation or mixed cropping can improve the fertility of the land.
viii. Solid Waste Treatment:
Paper methods should be adopted for management of solid waste disposal. Industrial wastes can be treated physically, chemically and biologically until they are less hazardous. Acidic and alkaline wastes should be first neutralized; the insoluble material if biodegradable should be allowed to degrade under controlled conditions before being disposed.
As a lost resort, new areas for storage of hazardous waste should be investigated such as deep well injection and more secure landfills. Burying the waste in locations situated away from residential areas is the simplest and most widely used technique of solid waste management. Environmental and aesthetic considerations must be taken into consideration before selecting the dumping sites.
Incineration of other wastes is expensive and leaves a huge residue and adds to air pollution. Pyrolysis is a process of combustion in absence of oxygen or the material burnt under controlled atmosphere of oxygen. It is an alternative to incineration.
The gas and liquid thus obtained can be used as fuels. Pyrolysis of carbonaceous wastes like firewood, coconut, palm waste, corn combs, cashew shell, rice husk paddy straw and saw dust, yields charcoal along with products like tar, methyl alcohol, acetic acid, acetone and a fuel gas.